An iron rheostat controls hematopoietic stem cell fate
- PMID: 38402617
- PMCID: PMC10939794
- DOI: 10.1016/j.stem.2024.01.011
An iron rheostat controls hematopoietic stem cell fate
Abstract
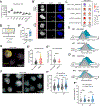
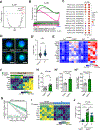
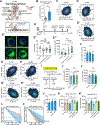
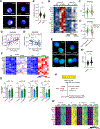
Mechanisms governing the maintenance of blood-producing hematopoietic stem and multipotent progenitor cells (HSPCs) are incompletely understood, particularly those regulating fate, ensuring long-term maintenance, and preventing aging-associated stem cell dysfunction. We uncovered a role for transitory free cytoplasmic iron as a rheostat for adult stem cell fate control. We found that HSPCs harbor comparatively small amounts of free iron and show the activation of a conserved molecular response to limited iron-particularly during mitosis. To study the functional and molecular consequences of iron restriction, we developed models allowing for transient iron bioavailability limitation and combined single-molecule RNA quantification, metabolomics, and single-cell transcriptomic analyses with functional studies. Our data reveal that the activation of the limited iron response triggers coordinated metabolic and epigenetic events, establishing stemness-conferring gene regulation. Notably, we find that aging-associated cytoplasmic iron loading reversibly attenuates iron-dependent cell fate control, explicating intervention strategies for dysfunctional aged stem cells.
Keywords: Tip60/KAT5; aging; gene regulation; hematopoiesis; iron; metabolism; stem cells.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.W. and U.S. have received funds for research projects and for serving on the advisory board of Novartis Pharmaceuticals.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

