Ginger alleviates mechanical hypersensitivity and anxio-depressive behavior in rats with diabetic neuropathy through beneficial actions on gut microbiome composition, mitochondria, and neuroimmune cells of colon and spinal cord
- PMID: 38402829
- PMCID: PMC11466295
- DOI: 10.1016/j.nutres.2024.01.014
Ginger alleviates mechanical hypersensitivity and anxio-depressive behavior in rats with diabetic neuropathy through beneficial actions on gut microbiome composition, mitochondria, and neuroimmune cells of colon and spinal cord
Abstract
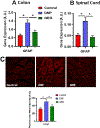
The relationship among gut microbiota, mitochondrial dysfunction/neuroinflammation, and diabetic neuropathic pain (DNP) has received increased attention. Ginger has antidiabetic and analgesic effects because of its anti-inflammatory property. We examined the effects of gingerols-enriched ginger (GEG) supplementation on pain-associated behaviors, gut microbiome composition, and mitochondrial function and neuroinflammation of colon and spinal cord in DNP rats. Thirty-three male rats were randomly divided into 3 groups: control group, DNP group (high-fat diet plus single dose of streptozotocin at 35 mg/kg body weight, and GEG group (DNP+GEG at 0.75% in the diet for 8 weeks). Von Frey and open field tests were used to assess pain sensitivity and anxio-depressive behaviors, respectively. Colon and spinal cord were collected for gene expression analysis. 16S rRNA gene sequencing was done from cecal samples and microbiome data analysis was performed using QIIME 2. GEG supplementation mitigated mechanical hypersensitivity and anxio-depressive behavior in DNP animals. GEG supplementation suppressed the dynamin-related protein 1 protein expression (colon) and gene expression (spinal cord), astrocytic marker GFAP gene expression (colon and spinal cord), and tumor necrosis factor-α gene expression (colon, P < .05; spinal cord, P = .0974) in DNP rats. GEG supplementation increased microglia/macrophage marker CD11b gene expression in colon and spinal cord of DNP rats. GEG treatment increased abundance of Acinetobacter, Azospirillum, Colidextribacter, and Fournierella but decreased abundance of Muribaculum intestinale in cecal feces of rats. This study demonstrates that GEG supplementation decreased pain, anxio-depression, and neuroimmune cells, and improved the composition of gut microbiomes and mitochondrial function in rats with diabetic neuropathy.
Keywords: Diabetic neuropathy; Ginger root extract; Gut microbiome; Mitochondria; Neuroinflammation.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that there is no conflict of interest.
Figures







References
-
- Heltianu C Role of nitric oxide synthase family in diabetic neuropathy. J Diabetes Metab 2011;S:5. doi:10.4172/2155-6156.S5-002. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

