A biomarker-stratified comparison of top-down versus accelerated step-up treatment strategies for patients with newly diagnosed Crohn's disease (PROFILE): a multicentre, open-label randomised controlled trial
- PMID: 38402895
- PMCID: PMC11001594
- DOI: 10.1016/S2468-1253(24)00034-7
A biomarker-stratified comparison of top-down versus accelerated step-up treatment strategies for patients with newly diagnosed Crohn's disease (PROFILE): a multicentre, open-label randomised controlled trial
Erratum in
-
Correction to Lancet Gastroenterol Hepatol 2024; 9: 415-27.Lancet Gastroenterol Hepatol. 2025 Aug;10(8):e10. doi: 10.1016/S2468-1253(25)00203-1. Lancet Gastroenterol Hepatol. 2025. PMID: 40651481 Free PMC article. No abstract available.
Abstract
Background: Management strategies and clinical outcomes vary substantially in patients newly diagnosed with Crohn's disease. We evaluated the use of a putative prognostic biomarker to guide therapy by assessing outcomes in patients randomised to either top-down (ie, early combined immunosuppression with infliximab and immunomodulator) or accelerated step-up (conventional) treatment strategies.
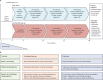
Methods: PROFILE (PRedicting Outcomes For Crohn's disease using a moLecular biomarker) was a multicentre, open-label, biomarker-stratified, randomised controlled trial that enrolled adults with newly diagnosed active Crohn's disease (Harvey-Bradshaw Index ≥7, either elevated C-reactive protein or faecal calprotectin or both, and endoscopic evidence of active inflammation). Potential participants had blood drawn to be tested for a prognostic biomarker derived from T-cell transcriptional signatures (PredictSURE-IBD assay). Following testing, patients were randomly assigned, via a secure online platform, to top-down or accelerated step-up treatment stratified by biomarker subgroup (IBDhi or IBDlo), endoscopic inflammation (mild, moderate, or severe), and extent (colonic or other). Blinding to biomarker status was maintained throughout the trial. The primary endpoint was sustained steroid-free and surgery-free remission to week 48. Remission was defined by a composite of symptoms and inflammatory markers at all visits. Flare required active symptoms (HBI ≥5) plus raised inflammatory markers (CRP >upper limit of normal or faecal calprotectin ≥200 μg/g, or both), while remission was the converse-ie, quiescent symptoms (HBI <5) or resolved inflammatory markers (both CRP ≤ the upper limit of normal and calprotectin <200 μg/g) or both. Analyses were done in the full analysis (intention-to-treat) population. The trial has completed and is registered (ISRCTN11808228).
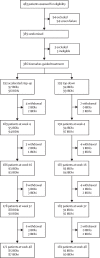
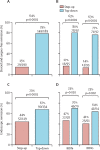
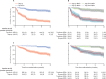
Findings: Between Dec 29, 2017, and Jan 5, 2022, 386 patients (mean age 33·6 years [SD 13·2]; 179 [46%] female, 207 [54%] male) were randomised: 193 to the top-down group and 193 to the accelerated step-up group. Median time from diagnosis to trial enrolment was 12 days (range 0-191). Primary outcome data were available for 379 participants (189 in the top-down group; 190 in the accelerated step-up group). There was no biomarker-treatment interaction effect (absolute difference 1 percentage points, 95% CI -15 to 15; p=0·944). Sustained steroid-free and surgery-free remission was significantly more frequent in the top-down group than in the accelerated step-up group (149 [79%] of 189 patients vs 29 [15%] of 190 patients, absolute difference 64 percentage points, 95% CI 57 to 72; p<0·0001). There were fewer adverse events (including disease flares) and serious adverse events in the top-down group than in the accelerated step-up group (adverse events: 168 vs 315; serious adverse events: 15 vs 42), with fewer complications requiring abdominal surgery (one vs ten) and no difference in serious infections (three vs eight).
Interpretation: Top-down treatment with combination infliximab plus immunomodulator achieved substantially better outcomes at 1 year than accelerated step-up treatment. The biomarker did not show clinical utility. Top-down treatment should be considered standard of care for patients with newly diagnosed active Crohn's disease.
Funding: Wellcome and PredictImmune Ltd.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests NMN reports personal fees from Galapagos, Janssen, Lilly, SBK Healthcare, and Takeda outside the submitted work; and grants from Dr Falk, Pfizer, Pharmacosmos, and Tillotts Pharma outside the submitted work. JCL reports consultancy fees from AbbVie, AgPlus Diagnostics, PredictImmune, and C4X Discover outside the submitted work; and grants from GSK outside the submitted work. KVP reports personal fees from AbbVie, DrFalk, Galapagos, Janssen, PredictImmune, Pfizer, and Takeda outside the submitted work; grants from AbbVie, Celltrion, Dr Falk, Ferring, Janssen, Takeda, and Tillotts Pharma outside the submitted work; and fees from advisory board membership of AbbVie, Galapagos, Janssen, and Pfizer outside the submitted work. TA reports personal fees from Amgen, Celltrion, Janssen, Lilly, Pfizer, and Tillotts Pharma; and grants from Biogen, Celltrion, Galapagos, Nova Pharmaceuticals, Pfizer, Roche, and Takeda outside the submitted work. JWB reports personal fees from Janssen outside the submitted work. RC reports personal fees from AbbVie, Galapagos, and Lilly outside the submitted work, grants from Celltrion, Ferring, and Tillotts Pharma outside the submitted work; and fees from advisory board membership of AbbVie and Bristol Myers Squibb outside the submitted work. SD reports personal fees from AbbVie, Ferring, Janssen, and Takeda outside the submitted work; grants from AbbVie, Dr Falk, and Janssen, outside the submitted work; and fees from advisory board membership of AbbVie outside the submitted work. DD reports personal fees from Takeda outside the submitted work; and grants from AbbVie and Janssen outside the submitted work. PMI reports personal fees from AbbVie, Arena, Boehringer-Ingelheim, BMS, Celgene, Celltrion, Dr Falk, Galapagos, Genentech, Gilead, Hospira, Janssen, Lilly, MSD, Pfizer, Prometheus, Sandoz, Samsung Bioepis, Sapphire Medical, Takeda, Topivert, VH2, Vifor Pharma, and Warner Chilcott outside the submitted work; and grants from AbbVie and Takeda outside the submitted work. AJK reports personal fees from AbbVie, Galapagos, and Takeda outside the submitted work; grants from AbbVie, Janssen, and Tillotts Pharma outside the submitted work; and fees from advisory board membership of AbbVie and Janssen outside the submitted work. CM reports grants from Janssen outside the submitted work. CSP reports personal fees from Dr Falk outside the submitted work. TR reports personal fees from AbbVie, Arena, Aslan, AstraZeneca, Boehringer-Ingelheim, BMS, Eli Lilly, Ferring, Galapagos, Gilead, GSK, Heptares, LabGenius, Novartis, Numab, Janssen, Pfizer, Roche, Takeda, UCB, and XAP therapeutics outside the submitted work; grants from AbbVie outside the submitted work; and fees from data monitoring board membership of UCB outside the submitted work. SS reports personal fees from AbbVie, Celltrion, Dr Falk, Ipsen, Janssen, and Takeda outside the submitted work. HRTW reports fees from advisory board membership of Pfizer outside the submitted work. SV reports personal fees from AbbVie, Abivax, AbolerISPharma, AgomAb, Alimentiv (formerly Robarts Clinical Trials), Arena Pharmaceuticals, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Cytoki Pharma, Dr Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead, GSK, Hospira, Imidomics, Janssen, J&J, Lilly, Materia Prima, Mestag Therapeutics, MiroBio, Morphic, MrMHealth, MSD, Mundipharma, Pfizer, Prodigest, Progenity, Prometheus, Surrozen, Takeda, Theravance, Tillotts Pharma, VectivBio, Ventyx, and Zealand Pharma outside the submitted work; and grants from AbbVie, Galapagos, J&J, Pfizer, and Takeda outside the submitted work. VJ reports personal fees from AbbVie, Alimentiv (formerly Robarts Clinical Trials), Arena Pharmaceuticals, Asahi Kasei Pharma, Asieris, AstraZeneca, Avoro Capital, BMS, Celltrion, Endpoint Health, Enthera, Ferring, Flagship Pioneering, Fresenius Kabi, Galapagos, Gilde Healthcare, GSK, Genentech, Gilead, Innomar, JAMP, Janssen, Lilly, Merck, Metacrine, Mylan, Pandion, Pendopharm, Pfizer, Protagonist, Prometheus, Reistone Biopharma, Roche, Roivant, Sandoz, SCOPE, Second Genome, Sorriso Pharmaceuticals, Takeda, Teva, Topivert, Ventyx, and Vividion outside the submitted work; and fees from advisory board membership of AbbVie, Alimentiv (formerly Robarts Clinical Trials), Arena Pharmaceuticals, Asahi Kasei Pharma, Asieris, AstraZeneca, BMS, Celltrion, Ferring, Flagship Pioneering, Fresenius Kabi, Galapagos, Gilde Healthcare, GSK, Genentech, Gilead, Innomar, JAMP, Janssen, Lilly, Merck, Metacran, Mylan, Pandion, Pendopharm, Pfizer, Protagonist, Prometheus, Reistone Biopharma, Roche, Sandoz, SCOPE, Second Genome, Sorriso Pharmaceuticals, Takeda, Teva, Topivert, Ventyx, and Vividion outside the submitted work. GRDH reports personal fees from AbbVie, Alimentiv, AstraZeneca, Immunic, J&J, Lilly, Pfizer, Takeda, Tillotts, and Ventyx outside the submitted work; grants from AbbVie, BMS, Lilly, Pfizer, and Takeda outside the submitted work; and fees from advisory board membership of AstraZeneca, Galapagos, and Seres Health outside the submitted work. EFM reports being a co-founder and receiving consultancy fees from PredictImmune; and holds PredictImmune stock or stock options. PAL reports being a co-founder and receiving consultancy fees from PredictImmune; and holds PredictImmune stock or stock options. JOL reports personal fees from AbbVie, Atlantic Healthcare, Bristol Meyer Squibb, Celgene, Celltrion, Engytix, Eli Lilly, Ferring, Galapagos, Gilead, GSK, Janssen, MSD, Norgine, Pfizer, Shire, and Takeda outside the submitted work; and grants from AbbVie, Ferring, Gilead, Takeda, and Shire outside the submitted work. NAK reports personal fees from Amgen, Bristol Myers Squibb, Celltrion, Falk, Galapagos, Janssen, Pfizer, Pharmacosmos, Takeda, and Tillotts Pharma outside the submitted work; and reports data monitoring board membership for the BEACON study outside the submitted work. KGCS reports being a co-founder and receiving consultancy fees from Predictimmune; consultancy fees from GSK outside the submitted work; and holds PredictImmune stock or stock options. MP reports personal fees from Janssen and Takeda outside the submitted work; and grants from AstraZeneca, Galapagos, Gilead, and Pfizer outside the submitted work. All other authors declare no competing interests.
Figures
Comment in
-
Has the time come for a systematic top-down approach in Crohn's disease?Lancet Gastroenterol Hepatol. 2024 May;9(5):394-395. doi: 10.1016/S2468-1253(24)00073-6. Lancet Gastroenterol Hepatol. 2024. PMID: 38604192 No abstract available.
-
Treatment strategies and biomarkers in Crohn's disease: the PROFILE trial.Lancet Gastroenterol Hepatol. 2024 Jul;9(7):590-591. doi: 10.1016/S2468-1253(24)00120-1. Lancet Gastroenterol Hepatol. 2024. PMID: 38870963 No abstract available.
-
Treatment strategies and biomarkers in Crohn's disease: the PROFILE trial.Lancet Gastroenterol Hepatol. 2024 Jul;9(7):591-592. doi: 10.1016/S2468-1253(24)00082-7. Lancet Gastroenterol Hepatol. 2024. PMID: 38870964 No abstract available.
-
Treatment strategies and biomarkers in Crohn's disease: the PROFILE trial.Lancet Gastroenterol Hepatol. 2024 Jul;9(7):591. doi: 10.1016/S2468-1253(24)00112-2. Lancet Gastroenterol Hepatol. 2024. PMID: 38870965 No abstract available.
-
Treatment strategies and biomarkers in Crohn's disease: the PROFILE trial.Lancet Gastroenterol Hepatol. 2024 Jul;9(7):592. doi: 10.1016/S2468-1253(24)00077-3. Lancet Gastroenterol Hepatol. 2024. PMID: 38870967 No abstract available.
-
The need for affordable, pragmatic, investigator-led clinical trials of treatment strategies in inflammatory bowel disease.Lancet Gastroenterol Hepatol. 2024 Oct;9(10):900-903. doi: 10.1016/S2468-1253(24)00225-5. Epub 2024 Jul 31. Lancet Gastroenterol Hepatol. 2024. PMID: 39094586 No abstract available.
References
-
- Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8:244–250. - PubMed
-
- Kalman TD, Everhov H, Nordenvall C, et al. Decrease in primary but not in secondary abdominal surgery for Crohn's disease: nationwide cohort study, 1990–2014. Br J Surg. 2020;107:1529–1538. - PubMed
-
- Eberhardson M, Söderling JK, Neovius M, et al. Anti-TNF treatment in Crohn's disease and risk of bowel resection—a population based cohort study. Aliment Pharmacol Ther. 2017;46:589–598. - PubMed
-
- Panés J, López-Sanromán A, Bermejo F, et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn's disease. Gastroenterology. 2013;145:766. 764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

