High-throughput screening identifies small molecule inhibitors of thioesterase superfamily member 1: Implications for the management of non-alcoholic fatty liver disease
- PMID: 38403978
- PMCID: PMC10663673
- DOI: 10.1016/j.molmet.2023.101832
High-throughput screening identifies small molecule inhibitors of thioesterase superfamily member 1: Implications for the management of non-alcoholic fatty liver disease
Abstract
Objective: Thioesterase superfamily member 1 (Them1) is a long chain acyl-CoA thioesterase comprising two N-terminal HotDog fold enzymatic domains linked to a C-terminal lipid-sensing steroidogenic acute regulatory transfer-related (START) domain, which allosterically modulates enzymatic activity. Them1 is highly expressed in thermogenic adipose tissue, where it functions to suppress energy expenditure by limiting rates of fatty acid oxidation, and is induced markedly in liver in response to high fat feeding, where it suppresses fatty acid oxidation and promotes glucose production. Them1-/- mice are protected against non-alcoholic fatty liver disease (NAFLD), suggesting Them1 as a therapeutic target.
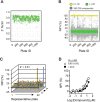
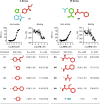
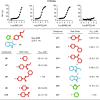
Methods: A high-throughput small molecule screen was performed to identify promising inhibitors targeting the fatty acyl-CoA thioesterase activity of purified recombinant Them1.Counter screening was used to determine specificity for Them1 relative to other acyl-CoA thioesterase isoforms. Inhibitor binding and enzyme inhibition were quantified by biophysical and biochemical approaches, respectively. Following structure-based optimization, lead compounds were tested in cell culture.
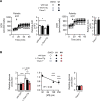
Results: Two lead allosteric inhibitors were identified that selectively inhibited Them1 by binding the START domain. In mouse brown adipocytes, these inhibitors promoted fatty acid oxidation, as evidenced by increased oxygen consumption rates. In mouse hepatocytes, they promoted fatty acid oxidation, but also reduced glucose production.
Conclusion: Them1 inhibitors could prove attractive for the pharmacologic management of NAFLD.
Keywords: Acyl-CoA thioesterase; Brown adipose tissue; Energy homeostasis; Insulin resistance; Lipid metabolism; Obesity associated metabolic disorders.
Copyright © 2023 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest D.E.C. has received research support from Sanofi in the form of an iAward. C.S.K, J.D.G. and D.E.C. have filed a patent application (No. 18/116,625) encompassing aspects of this work. The other authors declare no competing interests.
Figures




Similar articles
-
Thioesterase superfamily member 1 suppresses cold thermogenesis by limiting the oxidation of lipid droplet-derived fatty acids in brown adipose tissue.Mol Metab. 2016 Feb 23;5(5):340-351. doi: 10.1016/j.molmet.2016.02.002. eCollection 2016 May. Mol Metab. 2016. PMID: 27110486 Free PMC article.
-
Functional characterization of thioesterase superfamily member 1/Acyl-CoA thioesterase 11: implications for metabolic regulation.J Lipid Res. 2012 Dec;53(12):2620-31. doi: 10.1194/jlr.M029538. Epub 2012 Sep 19. J Lipid Res. 2012. PMID: 22993230 Free PMC article.
-
Targeted deletion of thioesterase superfamily member 1 promotes energy expenditure and protects against obesity and insulin resistance.Proc Natl Acad Sci U S A. 2012 Apr 3;109(14):5417-22. doi: 10.1073/pnas.1116011109. Epub 2012 Mar 16. Proc Natl Acad Sci U S A. 2012. PMID: 22427358 Free PMC article.
-
Regulation of energy metabolism by long-chain fatty acids.Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review.
-
Deactivating Fatty Acids: Acyl-CoA Thioesterase-Mediated Control of Lipid Metabolism.Trends Endocrinol Metab. 2017 Jul;28(7):473-484. doi: 10.1016/j.tem.2017.03.001. Epub 2017 Apr 3. Trends Endocrinol Metab. 2017. PMID: 28385385 Free PMC article. Review.
Cited by
-
Structure, function, and lipid sensing activity in the thioesterase superfamily.Biochem Soc Trans. 2024 Aug 28;52(4):1565-1577. doi: 10.1042/BST20230313. Biochem Soc Trans. 2024. PMID: 39140379 Free PMC article. Review.
-
ChREBP-mediated up-regulation of Them1 coordinates thermogenesis with glycolysis and lipogenesis in response to chronic stress.Sci Signal. 2024 Dec 3;17(865):eadk7971. doi: 10.1126/scisignal.adk7971. Epub 2024 Dec 3. Sci Signal. 2024. PMID: 39626011 Free PMC article.
References
-
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

