Fast, multicolour optical sectioning over extended fields of view with patterned illumination and machine learning
- PMID: 38404329
- PMCID: PMC10890859
- DOI: 10.1364/BOE.510912
Fast, multicolour optical sectioning over extended fields of view with patterned illumination and machine learning
Abstract
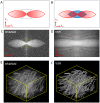
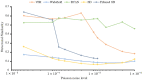
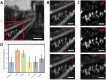
Structured illumination can reject out-of-focus signal from a sample, enabling high-speed and high-contrast imaging over large areas with widefield detection optics. However, this optical sectioning technique is currently limited by image reconstruction artefacts and poor performance at low signal-to-noise ratios. We combine multicolour interferometric pattern generation with machine learning to achieve high-contrast, real-time reconstruction of image data that is robust to background noise and sample motion. We validate the method in silico and demonstrate imaging of diverse specimens, from fixed and live biological samples to synthetic biosystems, reconstructing data live at 11 Hz across a 44 × 44μm2 field of view, and demonstrate image acquisition speeds exceeding 154 Hz.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Fast widefield imaging of neuronal structure and function with optical sectioning in vivo.Sci Adv. 2020 May 8;6(19):eaaz3870. doi: 10.1126/sciadv.aaz3870. eCollection 2020 May. Sci Adv. 2020. PMID: 32494711 Free PMC article.
-
Fast reconstruction and optical-sectioning three-dimensional structured illumination microscopy.Innovation (Camb). 2025 Jan 12;6(2):100757. doi: 10.1016/j.xinn.2024.100757. eCollection 2025 Feb 3. Innovation (Camb). 2025. PMID: 39991474 Free PMC article.
-
Flexible structured illumination microscope with a programmable illumination array.Opt Express. 2012 Oct 22;20(22):24585-99. doi: 10.1364/OE.20.024585. Opt Express. 2012. PMID: 23187221
-
Optical sectioning microscopy with planar or structured illumination.Nat Methods. 2011 Sep 29;8(10):811-9. doi: 10.1038/nmeth.1709. Nat Methods. 2011. PMID: 21959136 Review.
-
Grating imager systems for fluorescence optical-sectioning microscopy.Cold Spring Harb Protoc. 2014 Sep 2;2014(9):923-31. doi: 10.1101/pdb.top083493. Cold Spring Harb Protoc. 2014. PMID: 25183825 Review.
References
-
- Heintzmann R., Cremer C. G., “Laterally modulated excitation microscopy: improvement of resolution by using a diffraction grating,” in Optical Biopsies and Microscopic Techniques III , vol. 3568 (SPIE, 1999), pp. 185–196.
LinkOut - more resources
Full Text Sources
