A review of frustrated Lewis pair enabled monoselective C-F bond activation
- PMID: 38404400
- PMCID: PMC10882520
- DOI: 10.1039/d3sc06485a
A review of frustrated Lewis pair enabled monoselective C-F bond activation
Abstract
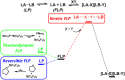
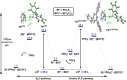
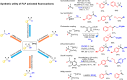
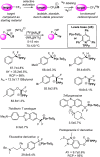
Frustrated Lewis pair (FLP) bond activation chemistry has greatly developed over the last two decades since the seminal report of metal-free reversible hydrogen activation. Recently, FLP systems have been utilized to allow monoselective C-F bond activation (at equivalent sites) in polyfluoroalkanes. The problem of 'over-defluorination' in the functionalization of polyfluoroalkanes (where multiple fluoro-positions are uncontrollably functionalized) has been a long-standing chemical problem in fluorocarbon chemistry for over 80 years. FLP mediated monoselective C-F bond activation is complementary to other solutions developed to address 'over-defluorination' and offers several advantages and unique opportunities. This perspective highlights some of these advantages and opportunities and places the development of FLP mediated C-F bond activation into the context of the wider effort to overcome 'over-defluorination'.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures










Similar articles
-
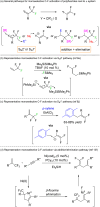
FLP-Catalyzed Monoselective C-F Functionalization in Polyfluorocarbons at Geminal or Distal Sites.Org Lett. 2021 Mar 5;23(5):1915-1920. doi: 10.1021/acs.orglett.1c00346. Epub 2021 Feb 24. Org Lett. 2021. PMID: 33624500
-
Structure-Reactivity Relationships in Borane-Based FLP-Catalyzed Hydrogenations, Dehydrogenations, and Cycloisomerizations.Acc Chem Res. 2023 Apr 4;56(7):821-834. doi: 10.1021/acs.accounts.2c00832. Epub 2023 Mar 13. Acc Chem Res. 2023. PMID: 36913645
-
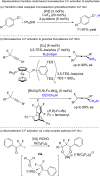
Stereoselective Synthesis of Fluoroalkanes via FLP Mediated Monoselective C─F Activation of Geminal Difluoroalkanes.Adv Sci (Weinh). 2023 Dec;10(36):e2305768. doi: 10.1002/advs.202305768. Epub 2023 Oct 31. Adv Sci (Weinh). 2023. PMID: 37907424 Free PMC article.
-
C-F Bond Activation Mediated by Phosphorus Compounds.Chemistry. 2019 Jul 17;25(40):9350-9357. doi: 10.1002/chem.201900542. Epub 2019 Mar 26. Chemistry. 2019. PMID: 30803077 Review.
-
On the concept of frustrated Lewis pairs.Philos Trans A Math Phys Eng Sci. 2017 Aug 28;375(2101):20170004. doi: 10.1098/rsta.2017.0004. Philos Trans A Math Phys Eng Sci. 2017. PMID: 28739963 Free PMC article. Review.
Cited by
-
Chemodivergent alkylation of trifluoromethyl alkenes via photocatalytic coupling with alkanes.Green Chem. 2024 Oct 4;26(22):11196-11205. doi: 10.1039/d4gc04176c. eCollection 2024 Nov 11. Green Chem. 2024. PMID: 39398964 Free PMC article.
-
Cryogenic Organometallic Carbon-Fluoride Bond Functionalization with Broad Functional Group Tolerance.J Am Chem Soc. 2025 Feb 19;147(7):5764-5774. doi: 10.1021/jacs.4c13956. Epub 2025 Feb 6. J Am Chem Soc. 2025. PMID: 39912296 Free PMC article.
-
Monodefluorinative Halogenation of Perfluoroalkyl Ketones via Organophosphorus-Mediated Selective C-F Activation.JACS Au. 2025 Jan 28;5(2):1007-1015. doi: 10.1021/jacsau.4c01242. eCollection 2025 Feb 24. JACS Au. 2025. PMID: 40017785 Free PMC article.
References
-
- Velders G. J. M. Ravishankara A. R. Miller M. K. Molina M. J. Alcamo J. Daniel J. S. Fahey D. W. Montzka S. A. Reimann S. Science. 2012;335:922. doi: 10.1126/science.1216414. - DOI - PubMed
- Montzka S. A. McFarland M. Andersen S. O. Miller B. R. Fahey D. W. Hall B. D. Hu L. Siso C. Elkins J. W. J. Phys. Chem. A. 2015;119:4439. doi: 10.1021/jp5097376. - DOI - PubMed
- Hurwitz M. M. Fleming E. L. Newman P. A. Li F. Mlawer E. Cady-Pereira K. Bailey R. Geophys. Res. Lett. 2015;42:8686. doi: 10.1002/2015GL065856. - DOI
- Fenton S. E. Ducatman A. Boobis A. DeWitt J. C. Lau C. Ng C. Smith J. S. Robertsh S. M. Environ. Toxicol. Chem. 2021;40:606. doi: 10.1002/etc.4890. - DOI - PMC - PubMed
- Dickman R. A. Aga D. S. J. Hazard. Mater. 2022;436:129120. doi: 10.1016/j.jhazmat.2022.129120. - DOI - PubMed
-
- Chandra G. Singh D. V. Mahato G. K. Patel S. Chem. Zvesti. 2023;13:1. - PMC - PubMed
- Barnes-Seeman D. Beck J. Springer C. Curr. Top. Med. Chem. 2014;14:855. doi: 10.2174/1568026614666140202204242. - DOI - PubMed
- Berger R. Resnati G. Metrangolo P. Weber E. Hulliger J. Chem. Soc. Rev. 2011;40:3496. doi: 10.1039/C0CS00221F. - DOI - PubMed
-
- Champagne P. A. Desroches J. Hamel J.-D. Vandamme M. Paquin J.-F. Chem. Rev. 2015;115:9073. doi: 10.1021/cr500706a. - DOI - PubMed
- Caron S. Org. Process Res. Dev. 2020;24:470. doi: 10.1021/acs.oprd.0c00030. - DOI
- Neumann C. N. Ritter T. Angew. Chem., Int. Ed. 2015;54:3216. doi: 10.1002/anie.201410288. - DOI - PubMed
- Bui T. T. Hong W. P. Kim H.-K. J. Fluorine Chem. 2021;247:109794. doi: 10.1016/j.jfluchem.2021.109794. - DOI
- Zou Z. Zhang W. Wang Y. Pan Y. Org. Chem. Front. 2021;8:2786. doi: 10.1039/D1QO00054C. - DOI
- Szpera R. Moseley D. F. J. Smith L. B. Sterling A. J. Gouverneur V. Angew. Chem., Int. Ed. 2019;58:14824. doi: 10.1002/anie.201814457. - DOI - PubMed
- Britton R. Gouverneur V. Lin J.-H. Meanwell M. Ni C. Pupo G. Xiao J.-C. Hu J. Nat. Rev. Methods Primers. 2021;1:47. doi: 10.1038/s43586-021-00042-1. - DOI
Publication types
LinkOut - more resources
Full Text Sources

