Nanoscale Luminescence Imaging/Detection of Single Particles: State-of-the-Art and Future Prospects
- PMID: 38404493
- PMCID: PMC10885340
- DOI: 10.1021/acsmeasuresciau.3c00052
Nanoscale Luminescence Imaging/Detection of Single Particles: State-of-the-Art and Future Prospects
Abstract
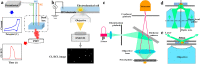
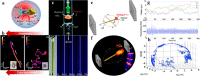
Single-particle-level measurements, during the reaction, avoid averaging effects that are inherent limitations of conventional ensemble strategies. It allows revealing structure-activity relationships beyond averaged properties by considering crucial particle-selective descriptors including structure/morphology dynamics, intrinsic heterogeneity, and dynamic fluctuations in reactivity (kinetics, mechanisms). In recent years, numerous luminescence (optical) techniques such as chemiluminescence (CL), electrochemiluminescence (ECL), and fluorescence (FL) microscopies have been emerging as dominant tools to achieve such measurements, owing to their diversified spectroscopy principles, noninvasive nature, higher sensitivity, and sufficient spatiotemporal resolution. Correspondingly, state-of-the-art methodologies and tools are being used for probing (real-time, operando, in situ) diverse applications of single particles in sensing, medicine, and catalysis. Herein, we provide a concise and comprehensive perspective on luminescence-based detection and imaging of single particles by putting special emphasis on their basic principles, mechanistic pathways, advances, challenges, and key applications. This Perspective focuses on the development of emission intensities and imaging based individual particle detection. Moreover, several key examples in the areas of sensing, motion, catalysis, energy, materials, and emerging trends in related areas are documented. We finally conclude with the opportunities and remaining challenges to stimulate further developments in this field.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Single-Particle Optical Imaging for Ultrasensitive Bioanalysis.Biosensors (Basel). 2022 Dec 1;12(12):1105. doi: 10.3390/bios12121105. Biosensors (Basel). 2022. PMID: 36551072 Free PMC article. Review.
-
Uncovering Photocatalytic Mechanisms toward Water Treatment by Operando Super-Resolution Reaction Imaging.Environ Sci Technol. 2025 May 27;59(20):9865-9885. doi: 10.1021/acs.est.5c00209. Epub 2025 Apr 30. Environ Sci Technol. 2025. PMID: 40304488 Review.
-
Recent developments in electrochemiluminescence nanosensors for cancer diagnosis applications.Nanoscale. 2020 Jul 14;12(26):13879-13898. doi: 10.1039/d0nr02844d. Epub 2020 Jun 24. Nanoscale. 2020. PMID: 32578649 Review.
-
Emerging Electrochemical Techniques for Probing Site Behavior in Single-Atom Electrocatalysts.Acc Chem Res. 2022 Mar 1;55(5):759-769. doi: 10.1021/acs.accounts.1c00785. Epub 2022 Feb 11. Acc Chem Res. 2022. PMID: 35148075
-
Electrochemiluminescence of Semiconductor Quantum Dots and Its Biosensing Applications: A Comprehensive Review.Biosensors (Basel). 2023 Jul 5;13(7):708. doi: 10.3390/bios13070708. Biosensors (Basel). 2023. PMID: 37504107 Free PMC article. Review.
Cited by
-
Single-particle Förster resonance energy transfer from upconversion nanoparticles to organic dyes.Nanoscale Adv. 2024 Apr 12;6(11):2945-2953. doi: 10.1039/d4na00198b. eCollection 2024 May 29. Nanoscale Adv. 2024. PMID: 38817426 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
