Blood-Brain Barrier Dysfunction Predicts Microglial Activation After Traumatic Brain Injury in Juvenile Rats
- PMID: 38404523
- PMCID: PMC10890961
- DOI: 10.1089/neur.2023.0057
Blood-Brain Barrier Dysfunction Predicts Microglial Activation After Traumatic Brain Injury in Juvenile Rats
Abstract
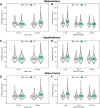
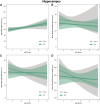
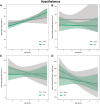
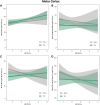
Traumatic brain injury (TBI) disrupts the blood-brain barrier (BBB), which may exacerbate neuroinflammation post-injury. Few translational studies have examined BBB dysfunction and subsequent neuroinflammation post-TBI in juveniles. We hypothesized that BBB dysfunction positively predicts microglial activation and that vulnerability to BBB dysfunction and associated neuroinflammation are dependent on age at injury. Post-natal day (PND)17 and PND35 rats (n = 56) received midline fluid percussion injury or sham surgery, and immunoglobulin-G (IgG) stain was quantified as a marker of extravasated blood in the brain and BBB dysfunction. We investigated BBB dysfunction and the microglial response in the hippocampus, hypothalamus, and motor cortex relative to age at injury and days post-injury (DPI; 1, 7, and 25). We measured the morphologies of ionized calcium-binding adaptor molecule 1-labeled microglia using cell body area and perimeter, microglial branch number and length, end-points/microglial cell, and number of microglia. Data were analyzed using generalized hierarchical models. In PND17 rats, TBI increased levels of IgG compared to shams. Independent of age at injury, IgG in TBI rats was higher at 1 and 7 DPI, but resolved by 25 DPI. TBI activated microglia (more cells and fewer end-points) in PND35 rats compared to respective shams. Independent of age at injury, TBI induced morphological changes indicative of microglial activation, which resolved by 25 DPI. TBI rats had fewer cells and end-points per cell at 1 and 7 DPI than 25 DPI. Independent of TBI, PND17 rats had larger, more activated microglia than PND35 rats; PND17 TBI rats had larger cell body areas and perimeters than PND35 TBI rats. Importantly, we found support in both ages that IgG quantification predicted microglial activation after TBI. The number of microglia increased with increasing IgG, whereas branch length decreased with increasing IgG, which together indicate microglial activation. Our results suggest that stabilization of the BBB after pediatric TBI may be an important therapeutic strategy to limit neuroinflammation and promote recovery.
Keywords: concussion; neuroinflammation; pediatric; vasculature.
© Tabitha R.F. Green et al., 2024; Published by Mary Ann Liebert, Inc.
Conflict of interest statement
No competing financial interests exist.
Figures





References
LinkOut - more resources
Full Text Sources
