Human metapneumovirus respiratory infection affects both innate and adaptive intestinal immunity
- PMID: 38404579
- PMCID: PMC10884822
- DOI: 10.3389/fimmu.2024.1330209
Human metapneumovirus respiratory infection affects both innate and adaptive intestinal immunity
Abstract
Introduction: Respiratory infections are one of the leading causes of morbidity and mortality worldwide, mainly in children, immunocompromised people, and the elderly. Several respiratory viruses can induce intestinal inflammation and alterations in intestinal microbiota composition. Human metapneumovirus (HMPV) is one of the major respiratory viruses contributing to infant mortality in children under 5 years of age worldwide, and the effect of this infection at the gut level has not been studied.
Methods: Here, we evaluated the distal effects of HMPV infection on intestinal microbiota and inflammation in a murine model, analyzing several post-infection times (days 1, 3, and 5). Six to eight-week-old C57BL/6 mice were infected intranasally with HMPV, and mice inoculated with a non-infectious supernatant (Mock) were used as a control group.
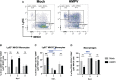
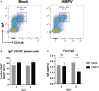
Results: We did not detect HMPV viral load in the intestine, but we observed significant changes in the transcription of IFN-γ in the colon, analyzed by qPCR, at day 1 post-infection as compared to the control group. Furthermore, we analyzed the frequencies of different innate and adaptive immune cells in the colonic lamina propria, using flow cytometry. The frequency of monocyte populations was altered in the colon of HMPV -infected mice at days 1 and 3, with no significant difference from control mice at day 5 post-infection. Moreover, colonic CD8+ T cells and memory precursor effector CD8+ T cells were significantly increased in HMPV-infected mice at day 5, suggesting that HMPV may also alter intestinal adaptive immunity. Additionally, we did not find alterations in antimicrobial peptide expression, the frequency of colonic IgA+ plasma cells, and levels of fecal IgA. Some minor alterations in the fecal microbiota composition of HMPV -infected mice were detected using 16s rRNA sequencing. However, no significant differences were found in β-diversity and relative abundance at the genus level.
Discussion: To our knowledge, this is the first report describing the alterations in intestinal immunity following respiratory infection with HMPV infection. These effects do not seem to be mediated by direct viral infection in the intestinal tract. Our results indicate that HMPV can affect colonic innate and adaptive immunity but does not significantly alter the microbiota composition, and further research is required to understand the mechanisms inducing these distal effects in the intestine.
Keywords: CD8 + T cells; HMPV; lung-gut axis; microbiota; monocytes.
Copyright © 2024 Sepúlveda-Alfaro, Catalán, Vallejos, Ramos-Tapia, Madrid-Muñoz, Mendoza-León, Suazo, Rivera-Asin, Silva, Alvarez-Mardones, Castillo-Godoy, Riedel, Schinnerling, Ugalde, Soto, Bueno, Kalergis and Melo-Gonzalez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




Similar articles
-
Lung CD8+ T Cell Impairment Occurs during Human Metapneumovirus Infection despite Virus-Like Particle Induction of Functional CD8+ T Cells.J Virol. 2015 Sep;89(17):8713-26. doi: 10.1128/JVI.00670-15. Epub 2015 Jun 10. J Virol. 2015. PMID: 26063431 Free PMC article.
-
Role of type I interferon signaling in human metapneumovirus pathogenesis and control of viral replication.J Virol. 2015 Apr;89(8):4405-20. doi: 10.1128/JVI.03275-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653440 Free PMC article.
-
Human Metapneumovirus: Mechanisms and Molecular Targets Used by the Virus to Avoid the Immune System.Front Immunol. 2018 Oct 24;9:2466. doi: 10.3389/fimmu.2018.02466. eCollection 2018. Front Immunol. 2018. PMID: 30405642 Free PMC article. Review.
-
T lymphocytes contribute to antiviral immunity and pathogenesis in experimental human metapneumovirus infection.J Virol. 2008 Sep;82(17):8560-9. doi: 10.1128/JVI.00699-08. Epub 2008 Jun 18. J Virol. 2008. PMID: 18562525 Free PMC article.
-
Aberrant T cell immunity triggered by human Respiratory Syncytial Virus and human Metapneumovirus infection.Virulence. 2017 Aug 18;8(6):685-704. doi: 10.1080/21505594.2016.1265725. Epub 2016 Dec 2. Virulence. 2017. PMID: 27911218 Free PMC article. Review.
Cited by
-
Genetically Predicted Peripheral Immune Cells Mediate the Effect of Gut Microbiota on Influenza Susceptibility.Int J Mol Sci. 2024 Jul 14;25(14):7706. doi: 10.3390/ijms25147706. Int J Mol Sci. 2024. PMID: 39062949 Free PMC article.
-
Intestinal-pulmonary axis: a 'Force For Good' against respiratory viral infections.Front Immunol. 2025 Mar 18;16:1534241. doi: 10.3389/fimmu.2025.1534241. eCollection 2025. Front Immunol. 2025. PMID: 40170840 Free PMC article. Review.
-
A Case of Severe Respiratory Failure Caused by Metapneumovirus and Influenza Virus in a Patient with HIV Infection.Viruses. 2025 Feb 20;17(3):289. doi: 10.3390/v17030289. Viruses. 2025. PMID: 40143221 Free PMC article.
-
hMPV Outbreaks: Worldwide Implications of a Re-Emerging Respiratory Pathogen.Microorganisms. 2025 Jun 27;13(7):1508. doi: 10.3390/microorganisms13071508. Microorganisms. 2025. PMID: 40732017 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

