Urchin-like multiscale structured fluorinated hydroxyapatite as versatile filler for caries restoration dental resin composites
- PMID: 38404640
- PMCID: PMC10885616
- DOI: 10.1016/j.bioactmat.2024.02.004
Urchin-like multiscale structured fluorinated hydroxyapatite as versatile filler for caries restoration dental resin composites
Abstract
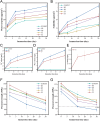
Caries is one of the most prevalent human diseases, resulting from demineralization of tooth hard tissue caused by acids produced from bacteria, and can progress to pulpal inflammation. Filling restoration with dental resin composites (DRCs) is currently the most common treatment for caries. However, existing DRCs suffer from low fracture strength and lack comprehensive anti-caries bioactivity including remineralization, pulp protection, and anti-cariogenic bacteria effects. In this study, inspired by plant roots' ability to stabilize and improve soil, fluorinated urchin-like hydroxyapatite (FUHA) with a three-dimensional whisker structure and bioactive components of calcium, phosphorus, and fluorine was designed and synthesized by a dynamic self-assembly method. Furthermore, versatile FUHA particles with different loading fractions were used as functional fillers to fabricate methacrylate-based DRCs, where the urchin-like hydroxyapatite (UHA) filled DRCs and commercial DRCs (Z350XT and BEAUTIFIL II) served as the control groups. The results demonstrated that FUHA with 50 wt% loading in resin matrix endowed DRC (F5) with excellent physicochemical properties, dentin remineralization property, cell viability, promotion of dental pulp stem cells mineralization, and antibacterial properties. Meanwhile, F5 also presented good clinical handling and aesthetic characteristics. Therefore, structure/functional-integrated FUHA filled DRCs have potential as a promising strategy for tooth restoration and anti-caries bioactivity.
Keywords: Anti-caries bioactivity; Dental resin composites; Micromechanical interlocking; Tooth remineralization; Versatile fluorinated hydroxyapatite.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- Love R.M., Tanner A.C.R. In: Microbiology of Dental Caries and Dentinal Tubule Infection. first ed. Fouad A.F., editor. John Wiley & Sons, Inc.; Hoboken: 2017. pp. 25–49. (Endod. Microbiol.). - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

