Investigating open access new approach methods (NAM) to assess biological points of departure: A case study with 4 neurotoxic pesticides
- PMID: 38404712
- PMCID: PMC10891343
- DOI: 10.1016/j.crtox.2024.100156
Investigating open access new approach methods (NAM) to assess biological points of departure: A case study with 4 neurotoxic pesticides
Abstract
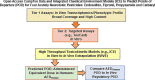
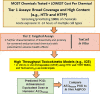
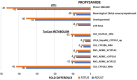
Open access new approach methods (NAM) in the US EPA ToxCast program and NTP Integrated Chemical Environment (ICE) were used to investigate activities of four neurotoxic pesticides: endosulfan, fipronil, propyzamide and carbaryl. Concordance of in vivo regulatory points of departure (POD) adjusted for interspecies extrapolation (AdjPOD) to modelled human Administered Equivalent Dose (AEDHuman) was assessed using 3-compartment or Adult/Fetal PBTK in vitro to in vivo extrapolation. Model inputs were from Tier 1 (High throughput transcriptomics: HTTr, high throughput phenotypic profiling: HTPP) and Tier 2 (single target: ToxCast) assays. HTTr identified gene expression signatures associated with potential neurotoxicity for endosulfan, propyzamide and carbaryl in non-neuronal MCF-7 and HepaRG cells. The HTPP assay in U-2 OS cells detected potent effects on DNA endpoints for endosulfan and carbaryl, and mitochondria with fipronil (propyzamide was inactive). The most potent ToxCast assays were concordant with specific components of each chemical mode of action (MOA). Predictive adult IVIVE models produced fold differences (FD) < 10 between the AEDHuman and the measured in vivo AdjPOD. The 3-compartment model was concordant (i.e., smallest FD) for endosulfan, fipronil and carbaryl, and PBTK was concordant for propyzamide. The most potent AEDHuman predictions for each chemical showed HTTr, HTPP and ToxCast were mainly concordant with in vivo AdjPODs but assays were less concordant with MOAs. This was likely due to the cell types used for testing and/or lack of metabolic capabilities and pathways available in vivo. The Fetal PBTK model had larger FDs than adult models and was less predictive overall.
Keywords: Carbaryl; Endosulfan; Fipronil; High Throughput Phenotypic Profile (HTPP); High Throughput Transcriptomics (HTTr); Integrated Chemical Environment (ICE); New Approach Methods (NAM); Propyzamide; ToxCast.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Use of computational toxicology models to predict toxicological points of departure: A case study with triazine herbicides.Birth Defects Res. 2023 Mar 15;115(5):525-544. doi: 10.1002/bdr2.2144. Epub 2022 Dec 30. Birth Defects Res. 2023. PMID: 36584090
-
A Comparison of ToxCast Test Results with In Vivo and Other In Vitro Endpoints for Neuro, Endocrine, and Developmental Toxicities: A Case Study Using Endosulfan and Methidathion.Birth Defects Res B Dev Reprod Toxicol. 2015 Apr;104(2):71-89. doi: 10.1002/bdrb.21140. Epub 2015 May 27. Birth Defects Res B Dev Reprod Toxicol. 2015. PMID: 26017137
-
Use of computational toxicology (CompTox) tools to predict in vivo toxicity for risk assessment.Regul Toxicol Pharmacol. 2020 Oct;116:104724. doi: 10.1016/j.yrtph.2020.104724. Epub 2020 Jul 5. Regul Toxicol Pharmacol. 2020. PMID: 32640296
-
Predictive models and computational toxicology.Methods Mol Biol. 2013;947:343-74. doi: 10.1007/978-1-62703-131-8_26. Methods Mol Biol. 2013. PMID: 23138916 Review.
-
Bridging the Data Gap From in vitro Toxicity Testing to Chemical Safety Assessment Through Computational Modeling.Front Public Health. 2018 Sep 11;6:261. doi: 10.3389/fpubh.2018.00261. eCollection 2018. Front Public Health. 2018. PMID: 30255008 Free PMC article. Review.
Cited by
-
RosetteArray® Platform for Quantitative High-Throughput Screening of Human Neurodevelopmental Risk.bioRxiv [Preprint]. 2024 Jun 20:2024.04.01.587605. doi: 10.1101/2024.04.01.587605. bioRxiv. 2024. PMID: 38798648 Free PMC article. Preprint.
-
Health Hazards Associated with Exposure to Endosulfan: A Mini-Review.Toxics. 2025 May 29;13(6):455. doi: 10.3390/toxics13060455. Toxics. 2025. PMID: 40559928 Free PMC article. Review.
References
-
- Abass, K., Turpeinen, M., Rautio, A., Hakkola, J., & Pelkonen, O. (2012). Metabolism of pesticides by human cytochrome P450 enzymes in vitro–a survey. Insecticides—advances in integrated pest management, 165-194.
-
- Abedini J., Cook B., Bell S.M., Chang X., Choksi N., Daniel A.B., Hines D., Karmaus A.L., Mansouri K., McAfee E., Phillips J., Rooney J., Sprankle C.S., Allen D.G., Casey W.M., Kleinstreuer N. Application of new approach methodologies: ICE tools to support chemical evaluations. Comput. Toxicol. 2021;20 doi: 10.1016/j.comtox.2021.100184. - DOI
-
- Andrus, A. K., & Hukkanen, R. R. (2011). Pronamide: Acute Neurotoxicity Study in F344/DuCrl Rats. Toxicology & Environmental Research and Consulting, The Dow Chemical Company, Midland, MI., Study ID: 101164, Chemical Code #: 694 ID #: SBRA 260827 E; DPR Vol. Record #: 317-0133 273313 EPA Reg. #: 62719-394 SB 950 #: 98(September 7, 2011).
LinkOut - more resources
Full Text Sources
Research Materials

