Role of IFITM2 in osteogenic differentiation of C3H10T1/2 mesenchymal stem cells
- PMID: 38404731
- PMCID: PMC10883848
- DOI: 10.5582/irdr.2023.01108
Role of IFITM2 in osteogenic differentiation of C3H10T1/2 mesenchymal stem cells
Abstract
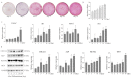
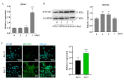
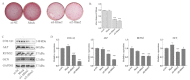
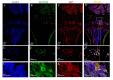
Interferon-inducible transmembrane (IFITM) are a family of small proteins localized to plasma and endolysosomal membranes. Their functions beyond restricting viral entry and replication have been revealed in recent years. IFITM5 is involved in bone mineralization and is an osteogenic cell surface marker. IFITM1 and 3 interact with desmin and myosin, and are involved in myogenic differentiation. This study found upregulation of Ifitm2 during osteogenic differentiation of C3H10T1/2 cells. This positively correlated to the expression of osteogenic differentiation markers Col1a1, Alp, Runx2, and Ocn. Knockdown of Ifitm2 by siRNAs inhibited osteogenic differentiation, calcium deposition, and osteogenic marker expression of C3H10T1/2 cells. The osteoblast transcriptome revealed that knocking down Ifitm2 affected the expression Wnt signaling pathway-related genes, including Wnt family members, their receptors Lrp, Frizzled, and Lgr, and transmembrane molecule Rnf43 that suppresses the Wnt signaling pathway. Luciferase assays indicated enhancement of canonical Wnt signaling pathways by Ifitm2 overexpression. Furthermore, IFITM2 was colocalized in the metaphyseal bone and growth plate of the mouse tibial bone with SP7, a transcription factor essential for osteoblast differentiation and bone formation. These findings reveal a possible novel function and potential mechanisms of Ifitm2 in osteogenic differentiation.
Keywords: C3H10T1/2 cells; IFITM2; TOP/FOP assay; Wnt/β-catenin signaling pathway; osteogenic differentiation.
2024, International Research and Cooperation Association for Bio & Socio - Sciences Advancement.
Conflict of interest statement
This work was supported by a grant from the Natural Science Foundation of Shandong Province (General program ZR2023MH276) and Academic Promotion Program of Shandong First Medical University (LJ001).The authors have no conflicts of interest to disclose.
Figures

Similar articles
-
Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway.Toxicol Lett. 2016 Jan 5;240(1):68-80. doi: 10.1016/j.toxlet.2015.10.007. Epub 2015 Oct 22. Toxicol Lett. 2016. PMID: 26478571
-
Ckip-1 regulates C3H10T1/2 mesenchymal cell proliferation and osteogenic differentiation via Lrp5.Exp Ther Med. 2021 Apr;21(4):342. doi: 10.3892/etm.2021.9773. Epub 2021 Feb 10. Exp Ther Med. 2021. PMID: 33732315 Free PMC article.
-
Wnt/β-catenin pathway regulates bone morphogenetic protein (BMP2)-mediated differentiation of dental follicle cells.J Periodontal Res. 2012 Jun;47(3):309-19. doi: 10.1111/j.1600-0765.2011.01433.x. Epub 2011 Dec 11. J Periodontal Res. 2012. PMID: 22150562 Free PMC article.
-
Phosphate promotes osteogenic differentiation through non-canonical Wnt signaling pathway in human mesenchymal stem cells.Bone. 2022 Nov;164:116525. doi: 10.1016/j.bone.2022.116525. Epub 2022 Aug 18. Bone. 2022. PMID: 35987514
-
Canonical Wnt signaling differently modulates osteogenic differentiation of mesenchymal stem cells derived from bone marrow and from periodontal ligament under inflammatory conditions.Biochim Biophys Acta. 2014 Mar;1840(3):1125-34. doi: 10.1016/j.bbagen.2013.11.003. Epub 2013 Nov 12. Biochim Biophys Acta. 2014. PMID: 24231680
Cited by
-
Revealing Fibrosis Genes as Biomarkers of Ulcerative Colitis: A Bioinformatics Study Based on ScRNA and Bulk RNA Datasets.Endocr Metab Immune Disord Drug Targets. 2025;25(9):710-720. doi: 10.2174/0118715303332155240912050838. Endocr Metab Immune Disord Drug Targets. 2025. PMID: 39428941
References
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143-147. - PubMed
-
- Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993; 14:424-442. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

