Drug Repurposing in Crohn's Disease Using Danish Real-World Data
- PMID: 38404739
- PMCID: PMC10894518
- DOI: 10.2147/POR.S444569
Drug Repurposing in Crohn's Disease Using Danish Real-World Data
Abstract
Aim: Drug repurposing, utilizing electronic healthcare records (EHRs), offers a promising alternative by repurposing existing drugs for new therapeutic indications, especially for patients lacking effective therapies. Intestinal fibrosis, a severe complication of Crohn's disease (CD), poses significant challenges, increasing morbidity and mortality without available pharmacological treatments. This article focuses on identifying medications associated with an elevated or reduced risk of fibrosis in CD patients through a population-wide real-world data and artificial intelligence (AI) approach.
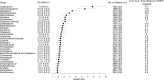
Methods: Patients aged 65 or older with a diagnosis of CD from 1996 to 2019 in the Danish EHRs were followed for up to 24 years. The primary outcome was the need of specific surgical procedures, namely proctocolectomy with ileostomy and ileocecal resection as proxies of intestinal fibrosis. The study explored drugs linked to an increased or reduced risk of the study outcome through machine-learning driven survival analysis.
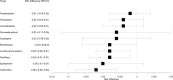
Results: Among the 9179 CD patients, 1029 (11.2%) underwent surgery, primarily men (58.5%), with a mean age of 76 years, 10 drugs were linked to an elevated risk of surgery for proctocolectomy with ileostomy and ileocecal resection. In contrast, 10 drugs were associated with a reduced risk of undergoing surgery for these conditions.
Conclusion: This study focuses on repurposing existing drugs to prevent surgery related to intestinal fibrosis in CD patients, using Danish EHRs and advanced statistical methods. The findings offer valuable insights into potential treatments for this condition, addressing a critical unmet medical need. Further research and clinical trials are warranted to validate the effectiveness of these repurposed drugs in preventing surgery related to intestinal fibrosis in CD patients.
Keywords: Crohn’s disease; drug repurposing; inflammatory bowel disease; intestinal fibrosis; machine-learning; real-world data.
© 2024 Shakibfar et al.
Conflict of interest statement
Dr Tine Jess reports personal fees from Ferring, outside the submitted work. Dr Julien Kirchgesner reports personal fees from Janssen, personal fees from Abbvie, personal fees from Pfizer, personal fees from Galapagos, personal fees from Takeda, personal fees from Tillots, personal fees from Amgen, personal fees from Lilly, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures



Similar articles
-
Quality of life and colorectal function in Crohn's disease patients that underwent ileocecal resection during childhood.Eur J Pediatr. 2019 Sep;178(9):1413-1421. doi: 10.1007/s00431-019-03427-3. Epub 2019 Jul 20. Eur J Pediatr. 2019. PMID: 31327075 Free PMC article.
-
A comprehensive review and update on Crohn's disease.Dis Mon. 2018 Feb;64(2):20-57. doi: 10.1016/j.disamonth.2017.07.001. Epub 2017 Aug 18. Dis Mon. 2018. PMID: 28826742 Review.
-
Colonic Crohn's disease - decision is more important than incision: A surgical dilemma.World J Gastrointest Surg. 2021 Jan 27;13(1):1-6. doi: 10.4240/wjgs.v13.i1.1. World J Gastrointest Surg. 2021. PMID: 33552390 Free PMC article. Review.
-
The MYTHS of De novo Crohn's Disease After Restorative Proctocolectomy with Ileal Pouch-anal Anastomosis for Ulcerative Colitis.Jpn J Gastroenterol Hepatol. 2020;3(2):1166. Epub 2020 Mar 11. Jpn J Gastroenterol Hepatol. 2020. PMID: 37584007 Free PMC article.
-
Small Bowel Crohn's Disease Recurrence is Common After Total Proctocolectomy for Crohn's Colitis.Dis Colon Rectum. 2022 Mar 1;65(3):390-398. doi: 10.1097/DCR.0000000000002328. Dis Colon Rectum. 2022. PMID: 34759246
Cited by
-
Systematic-Narrative Hybrid Literature Review: Crosstalk between Gastrointestinal Renin-Angiotensin and Dopaminergic Systems in the Regulation of Intestinal Permeability by Tight Junctions.Int J Mol Sci. 2024 May 20;25(10):5566. doi: 10.3390/ijms25105566. Int J Mol Sci. 2024. PMID: 38791603 Free PMC article. Review.
-
Drug Repurposing to Inhibit Oncostatin M in Crohn's Disease.Molecules. 2025 Apr 24;30(9):1897. doi: 10.3390/molecules30091897. Molecules. 2025. PMID: 40363705 Free PMC article.
-
Maximizing Treatment Options for IBD through Drug Repurposing.Curr Pharm Des. 2024;30(32):2538-2549. doi: 10.2174/0113816128318032240702045822. Curr Pharm Des. 2024. PMID: 39039672 Review.

