Short-Term Outcomes of 3 Monthly intravitreal Faricimab On Different Subtypes of Neovascular Age-Related Macular Degeneration
- PMID: 38405104
- PMCID: PMC10893793
- DOI: 10.2147/OPTH.S448507
Short-Term Outcomes of 3 Monthly intravitreal Faricimab On Different Subtypes of Neovascular Age-Related Macular Degeneration
Abstract
Purpose: To evaluate the efficacy and safety of faricimab injections for treatment-naïve neovascular age-related macular degeneration (nvAMD) patients, including subtypes and pachychoroid phenotypes, and identify predictive factors for visual outcomes.
Methods: nvAMD patients were prospectively recruited, receiving three monthly faricimab (6 mg) injections. Best-corrected visual acuity (BCVA) two months after the last injection (month 4) was compared between subtypes, and between pachychoroid neovasculopathy (PNV) and non-PNV eyes. Regression analysis determined factors influencing month 4 BCVA.
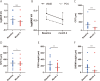
Results: The study involved 23 patients (12 typical AMD [tAMD], 10 polypoidal choroidal vasculopathy [PCV], 1 retinal angiomatous proliferation [RAP]). Eleven exhibited PNV phenotype. Significant BCVA (P = 4.9 × 10-4) and central retinal thickness (CRT) (P = 1.3 × 10-5) improvements were observed post-faricimab treatment. The therapy demonstrated favourable results for both tAMD and PCV eyes, and non-PNV and PNV eyes. Faricimab achieved dry macula in 77.3% of eyes, with subretinal fluid resolution in most cases, although intraretinal fluid (IRF) often persisted. Multivariable analysis identified external limiting membrane (ELM) presence and IRF as BCVA contributors at month 4.
Conclusion: Faricimab demonstrated significant effectiveness and safety in treatment-naïve nvAMD patients, particularly for PCV and PNV eyes. ELM presence and IRF is predictive of visual outcomes.
Keywords: CRT; ELM; IRF; MNV; PCV; PNV; RAP; anti-VEGF; anti-vascular endothelial growth factor; central retinal thickness; external limiting membrane; faricimab; intraretinal fluid; macular neovascularization; neovascular age-related macular degeneration; nvAMD; pachychoroid neovasculopathy; polypoidal choroidal vasculopathy; retinal angiomatous proliferation.
© 2024 Tanaka et al.
Conflict of interest statement
M Hata: Novartis Pharma, Senju Pharmaceutical, Alcon Research Institute, and Kyoto Drug Discovery and Development; A. N Ueda-Arakawa: Santen Pharmaceutical, Novartis Pharma, and Chugai Pharmaceutical; H. Tamura: Findex, Bayer Yakuhin, Novartis Pharma, Santen Pharmaceutical, SUNTORY, and Otsuka Pharmaceutical; M Miyata: Alcon Japan, Novartis Pharma, Santen Pharmaceutical, HOYA, Bayer Yakuhin, Senju Pharmaceutical, and Kowa Pharmaceutical; A. Takahashi: Bayer Yakuhin, Novartis Pharma, Santen Pharmaceutical, and MSD; Y. Muraoka: Rohto Pharmaceutical, Bayer Yakuhin, Novartis Pharma, Alcon Japan, Canon, Santen Pharmaceutical, Senju Pharmaceutical, AMO Japan, HOYA, Johnson & Johnson K.K.; M Miyake: Novartis Pharma, Bayer Yakuhin, Kowa Pharmaceutical, Alcon Japan, AMO Japan, Santen Pharmaceutical, Senju Pharmaceutical, Johnson and Johnson K. K., and Chugai Pharmaceutical; S. Ooto: Bayer Yakuhin, Kowa Pharmaceutical, Janssen Pharmaceutical, Novartis Pharma, AMO Japan, Santen Pharmaceutical, Alcon Japan, and Senju Pharmaceutical; A. Tsujikawa: Canon, Findex, Santen Pharmaceutical, Kowa Pharmaceutical, Pfizer, Sumitomo Pharma, Rhoto Pharmaceutical, Nippon Boehringer Ingelheim, Nidek, Johnson & Johnson, Nikon Solutions, AMO Japan, Senju Pharmaceutical, Wakamoto Pharmaceutical, Alcon Japan, Alcon Pharma, Otsuka Pharmaceutical, Tomey Corporation, Taiho Pharma, Hoya, Bayer Yakuhin, Novartis Pharma, Chugai Pharmaceutical, Astellas, Eisai, Daiich-Sankyo, Janssen Pharmaceutical, Kyoto Drug Discovery and Development, Allergan Japan, MSD, Ellex, HOYA, Sanwa Kagaku Kenkyusho, Nitten Pharmaceutical, and AbbVie GK. The authors report no other conflicts of interest in this work.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

