Cu2+-Citrate-Chitosan Complex Nanoparticles for the Chemodynamic Therapy of Lung Cancer
- PMID: 38405439
- PMCID: PMC10883013
- DOI: 10.1021/acsomega.3c09619
Cu2+-Citrate-Chitosan Complex Nanoparticles for the Chemodynamic Therapy of Lung Cancer
Abstract
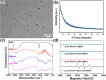
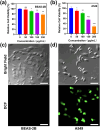
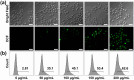
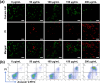
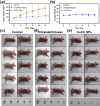
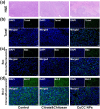
Lung cancer poses a significant threat to human health. Surgical intervention is the preferred treatment modality for lung cancer, but a large number of patients are deprived of the opportunity for surgery for various reasons and are compelled to undergo radiotherapy and chemotherapy, which entail systemic adverse reactions. In recent years, with the advancement of nanomedicine, chemodynamic therapy (CDT) based on free radicals has been extensively investigated. In this study, we fabricated copper-citrate-chitosan composite nanoparticles (CuCC NPs) by encapsulating copper-citrate complexes with natural chitosan polymers, resulting in a substantial reduction in the biotoxicity of copper ions. The CuCC NPs selectively accumulated in tumor tissues through the enhanced permeability and retention effect (EPR) and gradually degraded within the acidic and glutathione (GSH)-rich microenvironment of the tumor, thereby releasing the loaded copper ions. Through CDT, the copper ions converted the overexpressed hydrogen peroxide (H2O2) in the tumor tissue into hydroxyl radicals (•OH), leading to the eradication of tumor cells. In animal experiments, CuCC NPs exhibited remarkable efficacy in CDT. Further histopathological and hematological analyses demonstrated that CuCC NPs could induce substantial apoptosis in tumor tissues while maintaining an extremely high level of safety.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
pH-Activatable copper-axitinib coordinated multifunctional nanoparticles for synergistic chemo-chemodynamic therapy against aggressive cancers.Biomater Sci. 2023 Sep 12;11(18):6267-6279. doi: 10.1039/d3bm00861d. Biomater Sci. 2023. PMID: 37545202
-
H2O2 Self-Supplying and GSH-Depleting Nanocatalyst for Copper Metabolism-Based Synergistic Chemodynamic Therapy and Chemotherapy.Mol Pharm. 2023 Mar 6;20(3):1717-1728. doi: 10.1021/acs.molpharmaceut.2c00937. Epub 2023 Feb 21. Mol Pharm. 2023. PMID: 36809003
-
One-Pot Synthesis-Biocompatible Copper-Tripeptide Complex as a Nanocatalytic Medicine to Enhance Chemodynamic Therapy.ACS Biomater Sci Eng. 2021 Apr 12;7(4):1394-1402. doi: 10.1021/acsbiomaterials.0c01678. Epub 2021 Mar 9. ACS Biomater Sci Eng. 2021. PMID: 33689270
-
The Application of Biomedicine in Chemodynamic Therapy: From Material Design to Improved Strategies.Bioengineering (Basel). 2023 Aug 3;10(8):925. doi: 10.3390/bioengineering10080925. Bioengineering (Basel). 2023. PMID: 37627810 Free PMC article. Review.
-
Stimuli-activatable nanomedicines for chemodynamic therapy of cancer.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020 Jul;12(4):e1614. doi: 10.1002/wnan.1614. Epub 2020 Feb 3. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020. PMID: 32011108 Review.
Cited by
-
Advancing Therapeutic Strategies with Polymeric Drug Conjugates for Nucleic Acid Delivery and Treatment.Int J Nanomedicine. 2025 Jan 4;20:25-52. doi: 10.2147/IJN.S429279. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39802382 Free PMC article. Review.
-
Design of pH-Responsive Nanomaterials Based on the Tumor Microenvironment.Int J Nanomedicine. 2025 Jan 18;20:705-721. doi: 10.2147/IJN.S504629. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39845771 Free PMC article. Review.
-
Novel ST1926 Nanoparticle Drug Formulation Enhances Drug Therapeutic Efficiency in Colorectal Cancer Xenografted Mice.Nanomaterials (Basel). 2024 Aug 23;14(17):1380. doi: 10.3390/nano14171380. Nanomaterials (Basel). 2024. PMID: 39269042 Free PMC article.
-
Multifunctional magnetic nanocapsules for dual delivery of siRNA and chemotherapy to MCF-7 cells (Breast cancer cells).Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun 27. doi: 10.1007/s00210-025-04381-8. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40576767
References
-
- El-Sherif A.; Gooding W. E.; Santos R.; Pettiford B.; Ferson P. F.; Fernando H. C.; Urda S. J.; Luketich J. D.; Landreneau R. J. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: A 13-year analysis. Annals of Thoracic Surgery 2006, 82, 408–416. 10.1016/j.athoracsur.2006.02.029. - DOI - PubMed
LinkOut - more resources
Full Text Sources
