MXene-Embedded Porous Carbon-Based Cu2O Nanocomposites for Non-Enzymatic Glucose Sensors
- PMID: 38405472
- PMCID: PMC10882672
- DOI: 10.1021/acsomega.3c09659
MXene-Embedded Porous Carbon-Based Cu2O Nanocomposites for Non-Enzymatic Glucose Sensors
Abstract
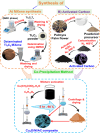
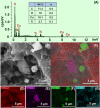
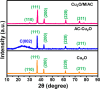
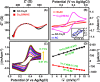
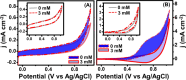
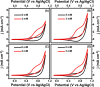
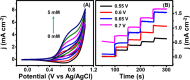
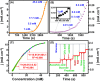
This work explores the use of MXene-embedded porous carbon-based Cu2O nanocomposite (Cu2O/M/AC) as a sensing material for the electrochemical sensing of glucose. The composite was prepared using the coprecipitation method and further analyzed for its morphological and structural characteristics. The highly porous scaffold of activated (porous) carbon facilitated the incorporation of MXene and copper oxide inside the pores and also acted as a medium for charge transfer. In the Cu2O/M/AC composite, MXene and Cu2O influence the sensing parameters, which were confirmed using electrochemical techniques such as cyclic voltammetry, electrochemical impedance spectroscopy, and amperometric analysis. The prepared composite shows two sets of linear ranges for glucose with a limit of detection (LOD) of 1.96 μM. The linear range was found to be 0.004 to 13.3 mM and 15.3 to 28.4 mM, with sensitivity values of 430.3 and 240.5 μA mM-1 cm-2, respectively. These materials suggest that the prepared Cu2O/M/AC nanocomposite can be utilized as a sensing material for non-enzymatic glucose sensors.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Dagogo-Jack S.Diabetes Mellitus in Developing Countries and Underserved Communities. Springer Cham; 2016, 1–294.10.1007/978-3-319-41559-8. - DOI
-
- Martinkova P.; Pohanka M. Biosensors for Blood Glucose and Diabetes Diagnosis: Evolution, Construction, and Current Status. Anal. Lett. 2015, 48 (16), 2509–2532. 10.1080/00032719.2015.1043661. - DOI
-
- Chaiyo S.; Mehmeti E.; Siangproh W.; Hoang T. L.; Nguyen H. P.; Chailapakul O.; Kalcher K. Non-Enzymatic Electrochemical Detection of Glucose with a Disposable Paper-Based Sensor Using a Cobalt Phthalocyanine–Ionic Liquid–Graphene Composite. Biosens. Bioelectron. 2018, 102, 113–120. 10.1016/j.bios.2017.11.015. - DOI - PubMed
LinkOut - more resources
Full Text Sources
