Design, Synthesis, and Evaluation of Novel Magnetic Nanoparticles Combined with Thiophene Derivatives for the Removal of Cr(VI) from an Aqueous Solution
- PMID: 38405514
- PMCID: PMC10883020
- DOI: 10.1021/acsomega.3c07517
Design, Synthesis, and Evaluation of Novel Magnetic Nanoparticles Combined with Thiophene Derivatives for the Removal of Cr(VI) from an Aqueous Solution
Abstract
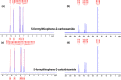
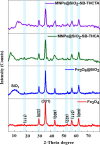
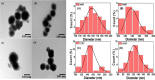
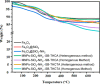
Most heavy metals are harmful to human health and the environment, even at extremely low concentrations. In natural waters, they are usually found only in trace amounts. Researchers are paying great attention to nanotechnology and nanomaterials as viable solutions to the problem of water pollution. This research focuses on the synthesis of organic thiophene derivatives that can be used as grafted ligands on the surface of silica-coated iron oxide nanoparticles to remove Cr(VI) chromium ions from water. The Vilsmeier-Haack reaction allows the formation of aldehyde groups in thiophene derivatives, and the resulting products were characterized by the FT-IR, NMR, and GC-MS. Schiff base is used as a binder between organic compounds and nanoparticles by the reaction of aldehyde groups in thiophene derivatives and amine groups on the surface of coated iron oxide nanoparticles. Schiff base functionalized Fe3O4 composites (MNPs@SiO2-SB-THCA) and (MNPs@SiO2-SB-THCTA) were successfully synthesized by homogeneous and heterogeneous methods and characterized by a combination of FT-IR, transmission electron microscopy, X-ray photoelectron spectroscopy, and thermogravimetric analysis. The adsorption studies, kinetic modeling, adsorption isotherms, and thermodynamics of the two materials, MNPs@SiO2-SB-THCA and MNPs@SiO2-SB-THCTA, were investigated for the removal of Cr(VI) from water at room temperature and at 50 mg/L. The high adsorption capacity at pH 6 for MNPs@SiO2-SB-THCTA was 15.53 mg/g, and for MNPs@SiO2-SB-THCA, it was 14.31 mg/g.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








Similar articles
-
Facile preparation of magnetic mesoporous MnFe2O4@SiO2-CTAB composites for Cr(VI) adsorption and reduction.Environ Pollut. 2017 Jan;220(Pt B):1376-1385. doi: 10.1016/j.envpol.2016.10.097. Epub 2016 Nov 9. Environ Pollut. 2017. PMID: 27836472
-
Surface functionalization of bamboo leave mediated synthesized SiO2 nanoparticles: Study of adsorption mechanism, isotherms and enhanced adsorption capacity for removal of Cr (VI) from aqueous solution.Environ Res. 2022 Nov;214(Pt 1):113761. doi: 10.1016/j.envres.2022.113761. Epub 2022 Jul 3. Environ Res. 2022. PMID: 35793724
-
Magnetite nanoparticles with aminomethylenephosphonic groups: synthesis, characterization and uptake of europium(III) ions from aqueous media.Mikrochim Acta. 2019 Jun 27;186(7):474. doi: 10.1007/s00604-019-3520-8. Mikrochim Acta. 2019. PMID: 31250114
-
One-Pot synthesis, characterization and adsorption studies of amine-functionalized magnetite nanoparticles for removal of Cr (VI) and Ni (II) ions from aqueous solution: kinetic, isotherm and thermodynamic studies.J Environ Health Sci Eng. 2016 Jul 26;14:11. doi: 10.1186/s40201-016-0252-0. eCollection 2016. J Environ Health Sci Eng. 2016. PMID: 27462402 Free PMC article.
-
The adsorption behavior and mechanism of Cr(VI) on facile synthesized mesoporous NH-SiO2.Environ Sci Pollut Res Int. 2020 Jan;27(3):2455-2463. doi: 10.1007/s11356-018-3599-1. Epub 2018 Nov 3. Environ Sci Pollut Res Int. 2020. PMID: 30392169
References
-
- Ethaib S.; Zubaidi S. L.. Removal of methylene blue dye from aqueous solution using kaolin. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 2nd International Scientific Conference of Al-Ayen University, Nasiriyah, Iraq, 15–16 July; IOP Publishing: Bristol, UK, 2020.
-
- Burakov A. E.; Galunin E. V.; Burakova I. V.; Kucherova A. E.; Agarwal S.; Tkachev A. G.; Gupta V. K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: a review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. 10.1016/j.ecoenv.2017.11.034. - DOI - PubMed
-
- Zulfiqar U.; Haider F. U.; Ahmad M.; Hussain S.; Maqsood M. F.; Ishfaq M.; Shahzad B.; Waqas M. M.; Ali B.; Tayyab M. N.; Ahmad S. A.; et al. Chromium toxicity, speciation, and remediation strategies in soil-plant interface: A critical review. Front. Plant Sci. 2023, 13, 1081624.10.3389/fpls.2022.1081624. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
