This is a preprint.
In vivo affinity maturation of the HIV-1 Env-binding domain of CD4
- PMID: 38405717
- PMCID: PMC10889057
- DOI: 10.21203/rs.3.rs-3922904/v1
In vivo affinity maturation of the HIV-1 Env-binding domain of CD4
Update in
-
In vivo affinity maturation of the CD4 domains of an HIV-1-entry inhibitor.Nat Biomed Eng. 2024 Dec;8(12):1715-1729. doi: 10.1038/s41551-024-01289-1. Epub 2024 Dec 5. Nat Biomed Eng. 2024. PMID: 39638875 Free PMC article.
Abstract
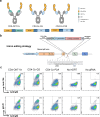
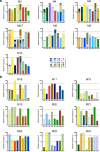
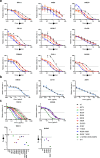
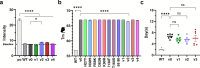
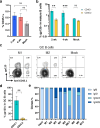
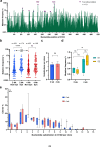
Many human proteins have been repurposed as biologics for clinical use. These proteins have been engineered with in vitro techniques that improve affinity for their ligands. However, these approaches do not select against properties that impair efficacy such as protease sensitivity or self-reactivity. Here we engineer the B-cell receptor of primary murine B cells to express a human protein biologic without disrupting their ability to affinity mature. Specifically, CD4 domains 1 and 2 (D1D2) of a half-life enhanced-HIV-1 entry inhibitor CD4-Ig (CD4-Ig-v0) were introduced into the heavy-chain loci of murine B cells, which were then adoptively transferred to wild-type mice. After immunization, transferred B cells proliferated, class switched, affinity matured, and efficiently produced D1D2-presenting antibodies. Somatic hypermutations found in the D1D2-encoding region of engrafted B cells improved binding affinity of CD4-Ig-v0 for the HIV-1 envelope glycoprotein (Env) and the neutralization potency of CD4-Ig-v0 by more than ten-fold across a global panel of HIV-1 isolates, without impairing its pharmacokinetic properties. Thus, affinity maturation of non-antibody protein biologics in vivo can guide development of more effective therapeutics.
Conflict of interest statement
COMPETING INTEREST A.P., W.H., T.O., Y.Y. and M.F. are inventors of a pending patent describing the in vivo affinity maturation of antibodies and biologics. C.C.B., M.D.A., and M.F. have equity stakes in Emmune, Inc., which developed CD4-Ig-v0. The authors have no other competing interests.
Figures











References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

