This is a preprint.
Myosin-I Synergizes with Arp2/3 Complex to Enhance Pushing Forces of Branched Actin Networks
- PMID: 38405741
- PMCID: PMC10888859
- DOI: 10.1101/2024.02.09.579714
Myosin-I Synergizes with Arp2/3 Complex to Enhance Pushing Forces of Branched Actin Networks
Update in
-
Myosin-I synergizes with Arp2/3 complex to enhance the pushing forces of branched actin networks.Sci Adv. 2024 Sep 13;10(37):eado5788. doi: 10.1126/sciadv.ado5788. Epub 2024 Sep 13. Sci Adv. 2024. PMID: 39270022 Free PMC article.
Abstract
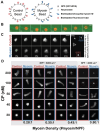
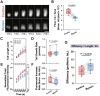
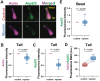
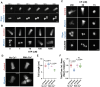
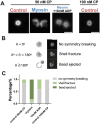
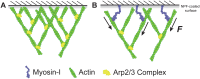
Myosin-Is colocalize with Arp2/3 complex-nucleated actin networks at sites of membrane protrusion and invagination, but the mechanisms by which myosin-I motor activity coordinates with branched actin assembly to generate force are unknown. We mimicked the interplay of these proteins using the "comet tail" bead motility assay, where branched actin networks are nucleated by Arp2/3 complex on the surface of beads coated with myosin-I and the WCA domain of N-WASP. We observed that myosin-I increased bead movement efficiency by thinning actin networks without affecting growth rates. Remarkably, myosin-I triggered symmetry breaking and comet-tail formation in dense networks resistant to spontaneous fracturing. Even with arrested actin assembly, myosin-I alone could break the network. Computational modeling recapitulated these observations suggesting myosin-I acts as a repulsive force shaping the network's architecture and boosting its force-generating capacity. We propose that myosin-I leverages its power stroke to amplify the forces generated by Arp2/3 complex-nucleated actin networks.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures


References
-
- Blanchoin L., Boujemaa-Paterski R., Sykes C., Plastino J., Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev 94, 235–263 (2014). - PubMed
-
- Rottner K., Faix J., Bogdan S., Linder S., Kerkhoff E., Actin assembly mechanisms at a glance. Journal of Cell Science 130, 3427–3435 (2017). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
