This is a preprint.
Revealing the atomic and electronic mechanism of human manganese superoxide dismutase product inhibition
- PMID: 38405788
- PMCID: PMC10889052
- DOI: 10.21203/rs.3.rs-3880128/v1
Revealing the atomic and electronic mechanism of human manganese superoxide dismutase product inhibition
Update in
-
Revealing the atomic and electronic mechanism of human manganese superoxide dismutase product inhibition.Nat Commun. 2024 Jul 16;15(1):5973. doi: 10.1038/s41467-024-50260-w. Nat Commun. 2024. PMID: 39013847 Free PMC article.
Abstract
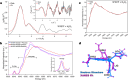
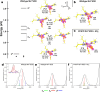
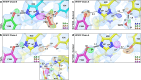
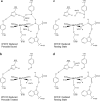
Human manganese superoxide dismutase (MnSOD) is a crucial oxidoreductase that maintains the vitality of mitochondria by converting to and with proton-coupled electron transfers (PCETs). Since changes in mitochondrial concentrations are capable of stimulating apoptotic signaling pathways, human MnSOD has evolutionarily gained the ability to be highly inhibited by its own product, . A separate set of PCETs is thought to regulate product inhibition, though mechanisms of PCETs are typically unknown due to difficulties in detecting the protonation states of specific residues that coincide with the electronic state of the redox center. To shed light on the underlying mechanism, we combined neutron diffraction and X-ray absorption spectroscopy of the product-bound, trivalent, and divalent states to reveal the all-atom structures and electronic configuration of the metal. The data identifies the product-inhibited complex for the first time and a PCET mechanism of inhibition is constructed.
Conflict of interest statement
Additional Declarations: There is NO Competing Interest.
Figures


Similar articles
-
Revealing the atomic and electronic mechanism of human manganese superoxide dismutase product inhibition.bioRxiv [Preprint]. 2024 Jan 27:2024.01.26.577433. doi: 10.1101/2024.01.26.577433. bioRxiv. 2024. Update in: Nat Commun. 2024 Jul 16;15(1):5973. doi: 10.1038/s41467-024-50260-w. PMID: 38328249 Free PMC article. Updated. Preprint.
-
Revealing the atomic and electronic mechanism of human manganese superoxide dismutase product inhibition.Nat Commun. 2024 Jul 16;15(1):5973. doi: 10.1038/s41467-024-50260-w. Nat Commun. 2024. PMID: 39013847 Free PMC article.
-
The role of Tyr34 in proton coupled electron transfer and product inhibition of manganese superoxide dismutase.Nat Commun. 2025 Feb 22;16(1):1887. doi: 10.1038/s41467-025-57180-3. Nat Commun. 2025. PMID: 39987263 Free PMC article.
-
MnSOD functions as a thermoreceptor activated by low temperature.J Inorg Biochem. 2022 Apr;229:111745. doi: 10.1016/j.jinorgbio.2022.111745. Epub 2022 Jan 29. J Inorg Biochem. 2022. PMID: 35121188 Review.
-
The O2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data.Acc Chem Res. 2017 Jan 17;50(1):41-48. doi: 10.1021/acs.accounts.6b00405. Epub 2016 Dec 21. Acc Chem Res. 2017. PMID: 28001034 Review.
References
-
- Albers D. S. & Beal M. F. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J. Neural Transm. Suppl. 59, 133–154 (2000). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

