This is a preprint.
Partitioned polygenic risk scores identify distinct types of metabolic dysfunction-associated steatotic liver disease
- PMID: 38405802
- PMCID: PMC10889080
- DOI: 10.21203/rs.3.rs-3878807/v1
Partitioned polygenic risk scores identify distinct types of metabolic dysfunction-associated steatotic liver disease
Update in
-
Partitioned polygenic risk scores identify distinct types of metabolic dysfunction-associated steatotic liver disease.Nat Med. 2024 Dec;30(12):3614-3623. doi: 10.1038/s41591-024-03284-0. Epub 2024 Dec 9. Nat Med. 2024. PMID: 39653778 Free PMC article.
Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) encompasses an excess of triglycerides in the liver, which can lead to cirrhosis and liver cancer. While there is solid epidemiological evidence of MASLD coexisting with cardiometabolic disease, several leading genetic risk factors for MASLD do not increase the risk of cardiovascular disease, suggesting no causal relationship between MASLD and cardiometabolic derangement. In this work, we leveraged measurements of visceral adiposity and identified 27 novel genetic loci associated with MASLD. Among these loci, we replicated 6 in several independent cohorts. Next, we generated two partitioned polygenic risk scores (PRS) based on the mechanism of genetic association with MASLD encompassing intra-hepatic lipoprotein retention. The two PRS suggest the presence of at least two distinct types of MASLD, one confined to the liver resulting in a more aggressive liver disease and one that is systemic and results in a higher risk of cardiometabolic disease.
Conflict of interest statement
Conflicts of Interest: S.R. has been consulting for AstraZeneca, GSK, Celgene Corporation, Ribo-cure AB and Pfizer in the last 5 years and received the research grant from AstraZeneca. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. L.V. has received speaking fees from MSD, Gilead, AlfaSigma and AbbVie, served as a consultant for Gilead, Pfizer, AstraZeneca, Novo Nordisk, Intercept, Diatech Pharmacogenetics, Ionis Pharmaceuticals, Boehringer Ingelheim, Resalis Therapeutics, and received unrestricted research grants from Gilead. R.L.G is a part-time contractor of Metabolon Inc. All other authors have none to declare.
Figures
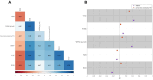
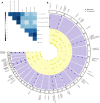
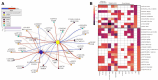



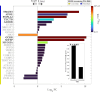
References
-
- Pais R., Redheuil A., Cluzel P., Ratziu V. & Giral P Relationship Among Fatty Liver, Specific and Multiple-Site Atherosclerosis, and 10-Year Framingham Score. Hepatology 69, 1453–1463 (2019). - PubMed
-
- Anstee Q.M., Targher G. & Day C. P Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 10, 330–344 (2013). - PubMed
-
- Toh J.Z.K., et al. A Meta-Analysis on the Global Prevalence, Risk factors and Screening of Coronary Heart Disease in Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 20, 2462–2473.e2410 (2022). - PubMed
-
- Romeo S., Sanyal A. & Valenti L. Leveraging Human Genetics to Identify Potential New Treatments for Fatty Liver Disease. Cell Metab 31, 35–45 (2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

