This is a preprint.
Repurposing mebendazole against triple-negative breast cancer leptomeningeal disease
- PMID: 38405839
- PMCID: PMC10889063
- DOI: 10.21203/rs.3.rs-3915392/v1
Repurposing mebendazole against triple-negative breast cancer leptomeningeal disease
Update in
-
Repurposing mebendazole against triple-negative breast cancer CNS metastasis.J Neurooncol. 2024 May;168(1):125-138. doi: 10.1007/s11060-024-04654-x. Epub 2024 Apr 2. J Neurooncol. 2024. PMID: 38563850 Free PMC article.
Abstract
Purpose: Triple-negative breast cancer (TNBC) is an aggressive subtype that often metastasizes to the brain. Leptomeningeal disease (LMD), a devastating brain metastasis common in TNBC, has limited treatment options. We sought to test whether the common anti-helminthic drug mebendazole (MBZ) may be effective against murine TNBC LMD.
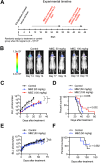
Methods: A small-molecule screen involving TNBC cell lines identified benzimidazoles as potential therapeutic agents for further study. In vitro migration assays were used to evaluate cell migration capacity and the effect of MBZ. For in vivo testing, LMD was introduced into BALB/c athymic nude mice through internal carotid artery injections of brain-tropic MDA-MB-231-BR or MCF7-BR cells. Tumor growth and spread was monitored by bioluminescence imaging. MBZ was given orally at 50 and 100 mg/kg doses. MBZ bioavailability was assayed by mass spectrometry.
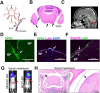
Results: Bioinformatic analysis and migration assays revealed higher migratory capacity of TNBC compared to other breast cancer subtypes. MBZ effectively slowed down migration of TNBC cell line MDA-MB-231 and its brain tropic derivative MDA-MB-231-BR. In animal studies, MBZ reduced tumor growth and extended survival in the LMD model produced by MDA-MB-231-BR cells. MBZ did not have an effect in the non-migratory MCF7-BR model.
Conclusions: We demonstrated that MBZ is a safe and effective oral agent in an animal model of TNBC LMD. Our findings are concordant with previous efforts involving MBZ and central nervous system pathology and further support the drug's potential utility as an alternative therapeutic for TNBC LMD.
Keywords: Breast cancer; drug repurposing; leptomeningeal disease; mebendazole.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

