This is a preprint.
Dorsal Raphe to Basolateral Amygdala Corticotropin-Releasing Factor Circuit Regulates Cocaine-Memory Reconsolidation
- PMID: 38405858
- PMCID: PMC10888894
- DOI: 10.1101/2024.02.10.579725
Dorsal Raphe to Basolateral Amygdala Corticotropin-Releasing Factor Circuit Regulates Cocaine-Memory Reconsolidation
Update in
-
Dorsal raphe to basolateral amygdala corticotropin-releasing factor circuit regulates cocaine-memory reconsolidation.Neuropsychopharmacology. 2024 Dec;49(13):2077-2086. doi: 10.1038/s41386-024-01892-5. Epub 2024 May 27. Neuropsychopharmacology. 2024. PMID: 38802479 Free PMC article.
Abstract
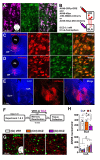
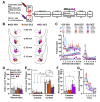
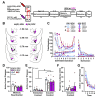
Environmental stimuli elicit drug craving and relapse in cocaine users by triggering the retrieval of strong cocainerelated contextual memories. Retrieval can also destabilize drug memories, requiring reconsolidation, a protein synthesis-dependent storage process, to maintain memory strength. Corticotropin-releasing factor (CRF) signaling in the basolateral amygdala (BLA) is necessary for cocainememory reconsolidation. We have hypothesized that a critical source of CRF in the BLA is the dorsal raphe nucleus (DR) based on its neurochemistry, anatomical connectivity, and requisite involvement in cocaine-memory reconsolidation. To test this hypothesis, male and female Sprague-Dawley rats received adeno-associated viruses to express Gi-coupled designer receptors exclusively activated by designer drugs (DREADDs) selectively in CRF neurons of the DR and injection cannulae directed at the BLA. The rats were trained to self-administer cocaine in a distinct environmental context then received extinction training in a different context. They were then briefly reexposed to the cocaine-predictive context to destabilize (reactivate) cocaine memories. Intra-BLA infusions of the DREADD agonist deschloroclozapine (DCZ; 0.1 mM, 0.5 μL/hemisphere) after memory reactivation attenuated cocaine-memory strength, relative to vehicle infusion. This was indicated by a selective, DCZ-induced and memory reactivation-dependent decrease in drug-seeking behavior in the cocaine-predictive context in DREADD-expressing males and females at test compared to respective controls. Notably, BLA-projecting DR CRF neurons that exhibited increased c-Fos expression during memory reconsolidation co-expressed glutamatergic and serotonergic neuronal markers. Together, these findings suggest that the DRCRF → BLA circuit is engaged to maintain cocaine-memory strength after memory destabilization, and this phenomenon may be mediated by DR CRF, glutamate, and/or serotonin release in the BLA.
Conflict of interest statement
Competing Interests The authors have nothing to disclose.
Figures


References
-
- Bouton ME, & Swartzentruber D. Analysis of the Associative and Occasion-Setting Properties of Contexts Participating in a Pavlovian Discrimination. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:333–50.
-
- O’brien CP, Childress A. R., Mclellan A. T., Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–15. - PubMed
-
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8(4):262–75. - PubMed
-
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47(6):795–801. - PubMed
-
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47(6):873–84. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
