Cystoid Macular Edema: A Rare Adverse Reaction to Rituximab
- PMID: 38406024
- PMCID: PMC10890713
- DOI: 10.7759/cureus.52867
Cystoid Macular Edema: A Rare Adverse Reaction to Rituximab
Abstract
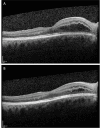
Membranous glomerulonephritis is the leading cause of nephrotic syndrome in non-diabetic Caucasian adults. For patients at risk of progressing to end-stage renal disease, immunosuppression, particularly rituximab, is the recommended treatment. While extremely rare, cases of cystoid macular edema associated with rituximab have been documented in the literature. In this report, we present the case of a 54-year-old male with membranous glomerulonephritis at a high risk of progressing to end-stage renal disease who experienced cystoid macular edema hours after receiving rituximab infusion. Following the discontinuation of the medication, the patient spontaneously recovered visual acuity without the need for any targeted therapy.
Keywords: adverse drug events; cystoid macular edema; membranous glomerulonephritis; nephrotic syndrome; rituximab.
Copyright © 2024, Machado et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Membranous nephropathy. Ronco P, Beck L, Debiec H, et al. Nat Rev Dis Primers. 2021;7:69. - PubMed
-
- KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. Kidney Int. 2021;100:0. - PubMed
-
- Rituximab - The first twenty years. Leandro M, Isenberg DA. Lupus. 2021;30:371–377. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
