Actives from the Micro-Immunotherapy Medicine 2LMIREG® Reduce the Expression of Cytokines and Immune-Related Markers Including Interleukin-2 and HLA-II While Modulating Oxidative Stress and Mitochondrial Function
- PMID: 38406323
- PMCID: PMC10894519
- DOI: 10.2147/JIR.S445053
Actives from the Micro-Immunotherapy Medicine 2LMIREG® Reduce the Expression of Cytokines and Immune-Related Markers Including Interleukin-2 and HLA-II While Modulating Oxidative Stress and Mitochondrial Function
Abstract
Introduction: Micro-immunotherapy (MI) is a therapeutic option employing low doses (LD) and ultra-low doses (ULD) of cytokines and immune factors to help the organism at modulating the immune responses. In an overpowering inflammatory context, this strategy may support the restoration of the body's homeostasis, as the active ingredients of MI medicines' (MIM) could boost or slow down the physiological functions of the immune cells. The aim of the study is to evaluate for the first time the in vitro anti-inflammatory properties of some actives employed by the MIM of interest in several human immune cell models.
Methods: In the first part of the study, the effects of the actives from the MIM of interest were assessed from a molecular standpoint: the expression of HLA-II, interleukin (IL)-2, and the secretion of several other cytokines were evaluated. In addition, as mitochondrial metabolism is also involved in the inflammatory processes, the second part of the study aimed at assessing the effects of these actives on the mitochondrial reactive oxygen species (ROS) production and on the mitochondrial membrane potential.
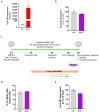
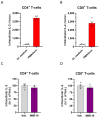
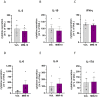
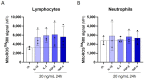
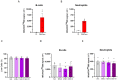
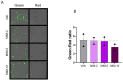
Results: We showed that the tested actives decreased the expression of HLA-DR and HLA-DP in IFN-γ-stimulated endothelial cells and in LPS-treated-M1-macrophages. The tested MIM slightly reduced the intracellular expression of IL-2 in CD4+ and CD8+ T-cells isolated from PMA/Iono-stimulated human PBMCs. Additionally, while the secretion of IL-2, IL-10, and IFN-γ was diminished, the treatment increased IL-6, IL-9, and IL-17A, which may correspond to a "Th17-like" secretory pattern. Interestingly, in PMA/Iono-treated PBMCs, we reported that the treatment reduced the ROS production in B-cells. Finally, in PMA/Iono-treated human macrophages, we showed that the treatment slightly protected the cells from early cell death/apoptosis.
Discussion: Overall, these results provide data about the molecular and functional anti-inflammatory effects of several actives contained in the tested MIM in immune-related cells, and their impact on two mitochondria-related processes.
Keywords: anti-inflammatory; cytokines; inflammation; interleukin-2; micro-immunotherapy; mitochondrial metabolism.
© 2024 Jacques et al.
Conflict of interest statement
The authors declared the following conflicts of interest with respect to the research, authorship, and/or publication of this article: Camille Jacques and Ilaria Floris work for Labo’Life France, the company service provider of Labo’Life, specialized in preclinical research and regulatory affairs. This professional relationship does not imply any misconduct on the part of the authors. Mathias Chatelais and Flora Marchand work for ProfileHIT, an innovative profiling company involved in vascular and immunology crosstalk research field in human. This study was entirely funded by Labolife France. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Special Focus on the Cellular Anti-Inflammatory Effects of Several Micro-Immunotherapy Formulations: Considerations Regarding Intestinal-, Immune-Axis-Related- and Neuronal-Inflammation Contexts.J Inflamm Res. 2022 Dec 13;15:6695-6717. doi: 10.2147/JIR.S389614. eCollection 2022. J Inflamm Res. 2022. PMID: 36536643 Free PMC article.
-
In Vitro Study of Interleukin-6 when Used at Low Dose and Ultra-Low Dose in Micro-Immunotherapy.Life (Basel). 2024 Mar 12;14(3):375. doi: 10.3390/life14030375. Life (Basel). 2024. PMID: 38541700 Free PMC article.
-
Active Substances from the Micro-Immunotherapy Medicine 2LC1® Show In Vitro Anti-Cancer Properties in Colon, Prostate, and Breast Cancer Models and Immune-Enhancing Capabilities in Human Macrophages.Int J Mol Sci. 2025 May 1;26(9):4300. doi: 10.3390/ijms26094300. Int J Mol Sci. 2025. PMID: 40362536 Free PMC article.
-
Ultra-Low Dose Cytokines in Rheumatoid Arthritis, Three Birds with One Stone as the Rationale of the 2LARTH® Micro-Immunotherapy Treatment.Int J Mol Sci. 2021 Jun 23;22(13):6717. doi: 10.3390/ijms22136717. Int J Mol Sci. 2021. PMID: 34201546 Free PMC article. Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
Understanding the Mode of Action of Several Active Ingredients from the Micro-Immunotherapy Medicine 2LZONA®.J Inflamm Res. 2025 Mar 21;18:4267-4290. doi: 10.2147/JIR.S498930. eCollection 2025. J Inflamm Res. 2025. PMID: 40134411 Free PMC article.
-
Exploring the Potential of Micro-Immunotherapy in the Treatment of Periodontitis.Life (Basel). 2024 Apr 25;14(5):552. doi: 10.3390/life14050552. Life (Basel). 2024. PMID: 38792574 Free PMC article. Review.
-
Kalanchoe tomentosa: Phytochemical Profiling, and Evaluation of Its Biological Activities In Vitro, In Vivo, and In Silico.Pharmaceuticals (Basel). 2024 Aug 9;17(8):1051. doi: 10.3390/ph17081051. Pharmaceuticals (Basel). 2024. PMID: 39204156 Free PMC article.
-
The Micro-Immunotherapy Medicine 2LPAPI® Displays Immune-Modulatory Effects in a Model of Human Papillomavirus Type-16 L1-Protein Capsid-Treated Human Peripheral Blood Mononuclear Cells and Antiproliferative Effects in a Model of Cervical Cancer Cells.Cancers (Basel). 2024 Apr 5;16(7):1421. doi: 10.3390/cancers16071421. Cancers (Basel). 2024. PMID: 38611099 Free PMC article.
-
Active Substances from the Micro-Immunotherapy Medicine 2LMIREG Display Antioxidative Properties In Vitro in Two Colorectal Cancer Cell Lines.Life (Basel). 2025 May 6;15(5):743. doi: 10.3390/life15050743. Life (Basel). 2025. PMID: 40430171 Free PMC article.
References
-
- López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13:106–118. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

