Identifying clinical response to daratumumab therapy in relapsed/refractory multiple myeloma using a patient-derived in vitro model
- PMID: 38406516
- PMCID: PMC10887349
- DOI: 10.1002/jha2.824
Identifying clinical response to daratumumab therapy in relapsed/refractory multiple myeloma using a patient-derived in vitro model
Abstract
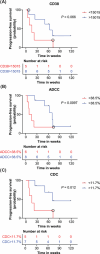
Response to daratumumab in patients with relapsed/refractory multiple myeloma is heterogeneous, and a reliable biomarker of response is lacking. We aimed to develop a method that identifies response to daratumumab therapy. Patient-derived MM cells were collected before start of daratumumab treatment and were cultured in a hydrogel-based culture system. The extent of antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity in vitro was associated with both clinical response and progression-free survival in corresponding patients. Together, our results demonstrate that in vitro sensitivity to daratumumab therapy in a hydrogel culture with primary MM cells might be used to identify patients most likely to benefit from treatment.
Keywords: daratumumab; multiple myeloma; patient‐derived plasma cells; relapsed/refractory multiple myeloma; response to therapy.
© 2023 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
M.C.M received honoraria paid to institution by Jansen Cilag, BMS, Gilead, Medscape, GSK, and Alnylam. M.J. received research funding by Novartis. V.P. received royalty payments related to venetoclax. The other authors declare no potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
