Prevalence and clinical impact of CD56 and T-cell marker expression in acute myeloid leukaemia: A single-centre retrospective analysis
- PMID: 38406551
- PMCID: PMC10887264
- DOI: 10.1002/jha2.827
Prevalence and clinical impact of CD56 and T-cell marker expression in acute myeloid leukaemia: A single-centre retrospective analysis
Abstract
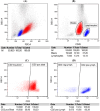
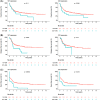
Flow cytometry-based immunophenotyping is a mainstay of diagnostics in acute myeloid leukaemia (AML). Aberrant CD56 and T-cell antigen expression is observed in a fraction subset of AML cases, but the clinical relevance remains incompletely understood. Here, we retrospectively investigated the association of CD56 and T-cell marker expression with disease-specific characteristics and outcome of 324 AML patients who received intensive induction therapy at our centre between 2011 and 2019. We found that CD2 expression was associated with abnormal non-complex karyotype, NPM1 wild-type status and TP53 mutation. CD2 also correlated with a lower complete remission (CR) rate (47.8% vs. 71.6%, p = 0.03). CyTdT and CD2 were associated with inferior 3-year event-free-survival (EFS) (5.3% vs. 33.5%, p = 0.003 and 17.4% vs. 33.1%, p = 0.02, respectively). CyTdT expression was also correlated with inferior relapse-free survival (27.3% vs. 48.8%, p = 0.04). In multivariable analyses CD2 positivity was an independent adverse factor for EFS (HR 1.72, p = 0.03). These results indicate a biological relevance of aberrant T-cell marker expression in AML and provide a rationale to further characterise the molecular origin in T-lineage-associated AML.
Keywords: CD56; T‐cell marker; acute myeloid leukaemia; clinical impact; flow cytometry.
© 2023 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Prognostic factors in normal karyotype acute myeloid leukemia in the absence of the FLT3-ITD mutation.Leuk Res. 2011 Apr;35(4):492-8. doi: 10.1016/j.leukres.2010.07.021. Epub 2010 Aug 3. Leuk Res. 2011. PMID: 20684989
-
[Immunophenotype characteristics and prognosis of acute leukemia patients with cross expressing lymphoid and myeloid lineage associated antigens].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010 Dec;18(6):1405-9. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010. PMID: 21176339 Chinese.
-
[Biological properties and sensitivity to induction therapy of differentiated cells expressing atypical immunophenotype in acute leukemia of children].Folia Med Cracov. 2001;42(3):5-80. Folia Med Cracov. 2001. PMID: 12353422 Review. Polish.
-
[Clinical Features and Prognosis of 227 cases of Acute Myeloid Leukemia with Cross-lineage Antigen Expression].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016 Aug;24(4):990-7. doi: 10.7534/j.issn.1009-2137.2016.04.006. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016. PMID: 27531762 Chinese.
-
The prognostic impact of tet oncogene family member 2 mutations in patients with acute myeloid leukemia: a systematic-review and meta-analysis.BMC Cancer. 2019 Apr 25;19(1):389. doi: 10.1186/s12885-019-5602-8. BMC Cancer. 2019. PMID: 31023266 Free PMC article.
References
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. New Engl J Med. 2015;373(12):1136–1152. - PubMed
-
- Kern W, Voskova D, Schoch C, Schnittger S, Hiddemann W, Haferlach T. Prognostic impact of early response to induction therapy as assessed by multiparameter flow cytometry in acute myeloid leukemia. Haematologica. 2004;89(5):528–540. - PubMed
-
- Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood. 2017;130(22):2373–2376. - PubMed
-
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
