Enhancing regeneration and repair of long-distance peripheral nerve defect injuries with continuous microcurrent electrical nerve stimulation
- PMID: 38406586
- PMCID: PMC10885699
- DOI: 10.3389/fnins.2024.1361590
Enhancing regeneration and repair of long-distance peripheral nerve defect injuries with continuous microcurrent electrical nerve stimulation
Abstract
Introduction: Peripheral nerve injuries, especially those involving long-distance deficits, pose significant challenges in clinical repair. This study explores the potential of continuous microcurrent electrical nerve stimulation (cMENS) as an adjunctive strategy to promote regeneration and repair in such cases.
Methods: The study initially optimized cMENS parameters and assessed its impact on Schwann cell activity, neurotrophic factor secretion, and the nerve regeneration microenvironment. Subsequently, a rat sciatic nerve defect-bridge repair model was employed to evaluate the reparative effects of cMENS as an adjuvant treatment. Functional recovery was assessed through gait analysis, motor function tests, and nerve conduction assessments. Additionally, nerve regeneration and denervated muscle atrophy were observed through histological examination.
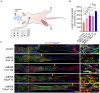
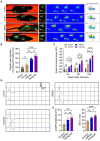
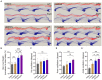
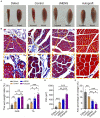
Results: The study identified a 10-day regimen of 100uA microcurrent stimulation as optimal. Evaluation focused on Schwann cell activity and the microenvironment, revealing the positive impact of cMENS on maintaining denervated Schwann cell proliferation and enhancing neurotrophic factor secretion. In the rat model of sciatic nerve defect-bridge repair, cMENS demonstrated superior effects compared to control groups, promoting motor function recovery, nerve conduction, and sensory and motor neuron regeneration. Histological examinations revealed enhanced maturation of regenerated nerve fibers and reduced denervated muscle atrophy.
Discussion: While cMENS shows promise as an adjuvant treatment for long-distance nerve defects, future research should explore extended stimulation durations and potential synergies with tissue engineering grafts to improve outcomes. This study contributes comprehensive evidence supporting the efficacy of cMENS in enhancing peripheral nerve regeneration.
Keywords: Schwann cell activity; continuous microcurrent electrical nerve stimulation; functional recovery; long-distance nerve defects; peripheral nerve injury; tissue engineering.
Copyright © 2024 Kong, Teng, Liu, Wang, Zhou, Zong, Wan, Qin, Yu, Mi and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Avendaño-Coy J., López-Muñoz P., Serrano-Muñoz D., Comino-Suárez N., Avendaño-López C., Martin-Espinosa N. (2022). Electrical microcurrent stimulation therapy for wound healing: a meta-analysis of randomized clinical trials. J. Tissue Viability 31, 268–277. doi: 10.1016/j.jtv.2021.12.002, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

