Rebastinib attenuates liver injury following cecal ligation and puncture in male mice
- PMID: 38406786
- PMCID: PMC10893574
- DOI: 10.25122/jml-2023-0089
Rebastinib attenuates liver injury following cecal ligation and puncture in male mice
Abstract
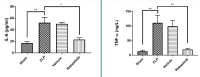
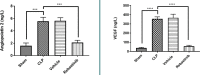
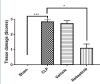
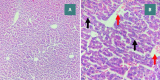
Sepsis remains a public health issue with high morbidity and mortality. Liver injury due to sepsis is associated with poor outcomes and an increased risk of death among septic patients. Rebastinib is a small molecule inhibiting the Tie2 receptor and vascular endothelial growth factor receptor-2 (VEGFR2). The current study aimed to reveal the potential protective impact of Rebastinib against sepsis-induced liver injury. Twenty-four adult male mice were allocated into four groups (six per group) as follows: Sham group was exposed to anesthesia and laparotomy with no cecal ligation and puncture procedure (CLP); CLP group was subjected CLP procedure; vehicle-treated group was pretreated with vehicle (oral route) one hour prior to CLP procedure; Rebastinib group was pretreated with oral Rebastinib one hour before induction of CLP. Collected blood was used to measure the serum levels of AST, ALT, and angiopoietin 2. Homogenized liver tissues were used to investigate IL-6, TNF-α, ICAM-1, MIF, VEGF, F2-isoprostanes, and caspase-11 levels. Histological examination was used to determine the severity of liver damage. Compared to the sham group, mice subjected to CLP had high levels of these biomarkers with a high degree of liver injury. In contrast, Rebastinib markedly reduced these levels and mitigated the liver damage. Rebastinib may be a hepatoprotective agent against sepsis-associated liver injury.
Keywords: Rebastinib-treated group; Sepsis; Tie2 receptor; inflammation; liver injury.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





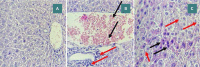
References
-
- Zhang P, Peng J, Ren Y-Q, Zheng H, Yan H. Dexmedetomidine protects against endothelial injury in septic rats induced by cecal ligation and puncture by decreasing angiopeiotin 2 and increasing vascular endothelial cadherin levels. Exp Ther Med. 2021;21:111. doi: 10.3892/etm.2020.9543. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
