Safety and reactogenicity of the BNT162b2 COVID-19 vaccine: Development, post-marketing surveillance, and real-world data
- PMID: 38407186
- PMCID: PMC10900268
- DOI: 10.1080/21645515.2024.2315659
Safety and reactogenicity of the BNT162b2 COVID-19 vaccine: Development, post-marketing surveillance, and real-world data
Abstract
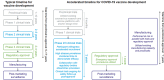
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to urgent actions by innovators, vaccine developers, regulators, and other stakeholders to ensure public access to protective vaccines while maintaining regulatory agency standards. Although development timelines for vaccines against SARS-CoV-2 were much quicker than standard vaccine development timelines, regulatory requirements for efficacy and safety evaluations, including the volume and quality of data collected, were upheld. Rolling review processes supported by sponsors and regulatory authorities enabled rapid assessment of clinical data as well as emergency use authorization. Post-authorization and pharmacovigilance activities enabled the quantity and breadth of post-marketing safety information to quickly exceed that generated from clinical trials. This paper reviews safety and reactogenicity data for the BNT162 vaccine candidates, including BNT162b2 (Comirnaty, Pfizer/BioNTech COVID-19 vaccine) and bivalent variant-adapted BNT162b2 vaccines, from preclinical studies, clinical trials, post-marketing surveillance, and real-world studies, including an unprecedentedly large body of independent evidence.
Keywords: SARS-CoV-2; post-marketing surveillance; reactogenicity; real-world studies; safety; vaccine development.
Conflict of interest statement
ÖT is a management board member and employee at BioNTech SE (Mainz, Germany) and co-founder of the company. FvO, NC, FJM, CL, SP, and RR are employees at BioNTech SE. ÖT is an inventor on patents and patent applications related to RNA technology and COVID-19 vaccines. SP, RR, CL and ÖT have securities from BioNTech SE.
Figures
References
-
- Vaccine Research and Development . [Internet]. Johns Hopkins University & Medicine; 2023. [accessed 2023 Mar 3]. https://coronavirus.jhu.edu/vaccines/timeline.
-
- Emergency use authorization for vaccines to prevent COVID-19: guidance for industry. [Internet]. US Food And Drug Administration; 2022. [accessed 2022 Jul 12]. https://www.fda.gov/media/142749/download.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous