DNA-PK controls Apollo's access to leading-end telomeres
- PMID: 38407308
- PMCID: PMC11077071
- DOI: 10.1093/nar/gkae105
DNA-PK controls Apollo's access to leading-end telomeres
Abstract
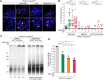
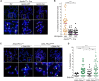
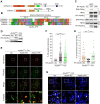
The complex formed by Ku70/80 and DNA-PKcs (DNA-PK) promotes the synapsis and the joining of double strand breaks (DSBs) during canonical non-homologous end joining (c-NHEJ). In c-NHEJ during V(D)J recombination, DNA-PK promotes the processing of the ends and the opening of the DNA hairpins by recruiting and/or activating the nuclease Artemis/DCLRE1C/SNM1C. Paradoxically, DNA-PK is also required to prevent the fusions of newly replicated leading-end telomeres. Here, we describe the role for DNA-PK in controlling Apollo/DCLRE1B/SNM1B, the nuclease that resects leading-end telomeres. We show that the telomeric function of Apollo requires DNA-PKcs's kinase activity and the binding of Apollo to DNA-PK. Furthermore, AlphaFold-Multimer predicts that Apollo's nuclease domain has extensive additional interactions with DNA-PKcs, and comparison to the cryo-EM structure of Artemis bound to DNA-PK phosphorylated on the ABCDE/Thr2609 cluster suggests that DNA-PK can similarly grant Apollo access to the DNA end. In agreement, the telomeric function of DNA-PK requires the ABCDE/Thr2609 cluster. These data reveal that resection of leading-end telomeres is regulated by DNA-PK through its binding to Apollo and its (auto)phosphorylation-dependent positioning of Apollo at the DNA end, analogous but not identical to DNA-PK dependent regulation of Artemis at hairpins.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
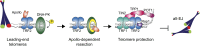



References
-
- Wu Q., Liang S., Ochi T., Chirgadze D.Y., Huiskonen J.T., Blundell T.L.. Understanding the structure and role of DNA-PK in NHEJ: how X-ray diffraction and cryo-EM contribute in complementary ways. Prog. Biophys. Mol. Biol. 2019; 147:26–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

