LTA4H inhibitor LYS006: Clinical PK/PD and safety in a randomized phase I clinical trial
- PMID: 38407540
- PMCID: PMC10837484
- DOI: 10.1111/cts.13724
LTA4H inhibitor LYS006: Clinical PK/PD and safety in a randomized phase I clinical trial
Abstract
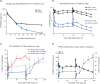
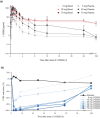
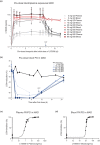
LYS006 is a novel, highly potent and selective, new-generation leukotriene A4 hydrolase (LTA4H) inhibitor in clinical development for the treatment of neutrophil-driven inflammatory diseases. We describe the complex pharmacokinetic to pharmacodynamic (PD) relationship in blood, plasma, and skin of LYS006-treated nonclinical species and healthy human participants. In a randomized first in human study, participants were exposed to single ascending doses up to 100 mg and multiple ascending doses up to 80 mg b.i.d.. LYS006 showed rapid absorption, overall dose proportional plasma exposure and nonlinear blood to plasma distribution caused by saturable target binding. The compound efficiently inhibited LTB4 production in human blood and skin blister cells, leading to greater than 90% predose target inhibition from day 1 after treatment initiation at doses of 20 mg b.i.d. and above. Slow re-distribution from target expressing cells resulted in a long terminal half-life and a long-lasting PD effect in ex vivo stimulated blood and skin cells despite low plasma exposures. LYS006 was well-tolerated and demonstrated a favorable safety profile up to highest doses tested, without any dose-limiting toxicity. This supported further clinical development in phase II studies in predominantly neutrophil-driven inflammatory conditions, such as hidradenitis suppurativa, inflammatory acne, and ulcerative colitis.
© 2024 Novartis Biomedical Research. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
We disclose that all authors associated with Novartis Biomedical Research or Novartis Pharma AG are current or former employees of and hold company stocks or stock options with Novartis Pharma AG, the company that is developing LYS006 as pharmacological treatment in inflammatory diseases. All other authors declared no competing interests for this work.
Figures

References
-
- Ford‐Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286(5770):264‐265. - PubMed
-
- Peters‐Golden M, Henderson WR Jr. Leukotrienes. N Engl J Med. 2007;357(18):1841‐1854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

