Bone Regeneration with Mesenchymal Stem Cells in Scaffolds: Systematic Review of Human Clinical Trials
- PMID: 38407793
- PMCID: PMC11087324
- DOI: 10.1007/s12015-024-10696-5
Bone Regeneration with Mesenchymal Stem Cells in Scaffolds: Systematic Review of Human Clinical Trials
Abstract
The aim of the study is to determine the effectiveness of stem cells in scaffolds in the treatment of bone deficits, in regard of bone regeneration, safety, rehabilitation and quality of life in humans. The systematic review was conducted in accordance with PRISMA 2020. A systematic search was conducted in three search engines and two registries lastly in 29-9-2022.for studies of the last 15 years. The risk of bias was assessed with RoB-2, ROBINS- I and NIH Quality of Before-After (Pre-Post) Studies with no Control group. The certainty of the results was assessed with the GRADE assessment tool. Due to heterogeneity, the results were reported in tables, graphs and narratively. The study protocol was published in PROSPERO with registration number CRD42022359049. Of the 10,091 studies retrieved, 14 were meeting the inclusion criteria, and were qualitatively analyzed. 138 patients were treated with mesenchymal stem cells in scaffolds, showing bone healing in all cases, and even with better results than the standard care. The adverse events were mild in most cases and in accordance with the surgery received. When assessed, there was a rehabilitation of the deficit and a gain in quality of life was detected. Although the heterogeneity between the studies and the small number of patients, the administration of mesenchymal stem cells in scaffolds seems safe and effective in the regeneration of bone defects. These results pave the way for the conduction of more clinical trials, with greater number of participants, with more standardized procedures.
Keywords: Biomaterial; Mesenchymal stem cell; Scaffold; Systematic review; bone.
© 2024. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare that are relevant to the content of this article.
The authors declare that they have no conflict of interest.
Figures

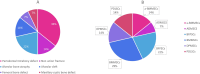

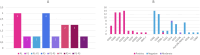




References
-
- Najar M, Melki R, Khalife F, Lagneaux L, Bouhtit F, Moussa Agha D, Fahmi H, Lewalle P, Fayyad-Kazan M, Merimi M. Therapeutic mesenchymal Stem/Stromal cells: Value, challenges and optimization. Frontiers in Cell and Developmental Biology. 2022;9(January):716853. doi: 10.3389/fcell.2021.716853. - DOI - PMC - PubMed
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

