Viral DNAemia and DNA Virus Seropositivity and Mortality in Pediatric Sepsis
- PMID: 38407904
- PMCID: PMC10897747
- DOI: 10.1001/jamanetworkopen.2024.0383
Viral DNAemia and DNA Virus Seropositivity and Mortality in Pediatric Sepsis
Erratum in
-
Errors in Figure.JAMA Netw Open. 2024 Mar 4;7(3):e249203. doi: 10.1001/jamanetworkopen.2024.9203. JAMA Netw Open. 2024. PMID: 38536178 Free PMC article. No abstract available.
Abstract
Importance: Sepsis is a leading cause of pediatric mortality. Little attention has been paid to the association between viral DNA and mortality in children and adolescents with sepsis.
Objective: To assess the association of the presence of viral DNA with sepsis-related mortality in a large multicenter study.
Design, setting, and participants: This cohort study compares pediatric patients with and without plasma cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus 1 (HSV-1), human herpesvirus 6 (HHV-6), parvovirus B19 (B19V), BK polyomavirus (BKPyV), human adenovirus (HAdV), and torque teno virus (TTV) DNAemia detected by quantitative real-time polymerase chain reaction or plasma IgG antibodies to CMV, EBV, HSV-1, or HHV-6. A total of 401 patients younger than 18 years with severe sepsis were enrolled from 9 pediatric intensive care units (PICUs) in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Data were collected from 2015 to 2018. Samples were assayed from 2019 to 2022. Data were analyzed from 2022 to 2023.
Main outcomes and measures: Death while in the PICU.
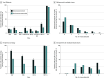
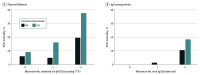
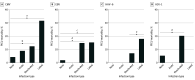
Results: Among the 401 patients included in the analysis, the median age was 6 (IQR, 1-12) years, and 222 (55.4%) were male. One hundred fifty-four patients (38.4%) were previously healthy, 108 (26.9%) were immunocompromised, and 225 (56.1%) had documented infection(s) at enrollment. Forty-four patients (11.0%) died in the PICU. Viral DNAemia with at least 1 virus (excluding TTV) was detected in 191 patients (47.6%) overall, 63 of 108 patients (58.3%) who were immunocompromised, and 128 of 293 (43.7%) who were not immunocompromised at sepsis onset. After adjustment for age, Pediatric Risk of Mortality score, previously healthy status, and immunocompromised status at sepsis onset, CMV (adjusted odds ratio [AOR], 3.01 [95% CI, 1.36-6.45]; P = .007), HAdV (AOR, 3.50 [95% CI, 1.46-8.09]; P = .006), BKPyV (AOR. 3.02 [95% CI, 1.17-7.34]; P = .02), and HHV-6 (AOR, 2.62 [95% CI, 1.31-5.20]; P = .007) DNAemia were each associated with increased mortality. Two or more viruses were detected in 78 patients (19.5%), with mortality among 12 of 32 (37.5%) who were immunocompromised and 9 of 46 (19.6%) who were not immunocompromised at sepsis onset. Herpesvirus seropositivity was common (HSV-1, 82 of 246 [33.3%]; CMV, 107 of 254 [42.1%]; EBV, 152 of 251 [60.6%]; HHV-6, 253 if 257 [98.4%]). After additional adjustment for receipt of blood products in the PICU, EBV seropositivity was associated with increased mortality (AOR, 6.10 [95% CI, 1.00-118.61]; P = .049).
Conclusions and relevance: The findings of this cohort study suggest that DNAemia for CMV, HAdV, BKPyV, and HHV-6 and EBV seropositivity were independently associated with increased sepsis mortality. Further investigation of the underlying biology of these viral DNA infections in children with sepsis is warranted to determine whether they only reflect mortality risk or contribute to mortality.
Conflict of interest statement
Figures

References
-
- Castellanos-Ortega A, Suberviola B, García-Astudillo LA, et al. . Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med. 2010;38(4):1036-1043. doi:10.1097/CCM.0b013e3181d455b6 - DOI - PubMed
-
- Irving SY, Daly B, Verger J, et al. ; Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network . The association of nutrition status expressed as body mass index z score with outcomes in children with severe sepsis: a secondary analysis from the Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study. Crit Care Med. 2018;46(11):e1029-e1039. doi:10.1097/CCM.0000000000003351 - DOI - PMC - PubMed
-
- Lin JC, Spinella PC, Fitzgerald JC, et al. ; Sepsis Prevalence, Outcomes, and Therapy Study Investigators . New or progressive multiple organ dysfunction syndrome in pediatric severe sepsis: a sepsis phenotype with higher morbidity and mortality. Pediatr Crit Care Med. 2017;18(1):8-16. doi:10.1097/PCC.0000000000000978 - DOI - PMC - PubMed

