Extracellular Vesicles Slow Down Aβ(1-42) Aggregation by Interfering with the Amyloid Fibril Elongation Step
- PMID: 38408014
- PMCID: PMC10921407
- DOI: 10.1021/acschemneuro.3c00655
Extracellular Vesicles Slow Down Aβ(1-42) Aggregation by Interfering with the Amyloid Fibril Elongation Step
Abstract
Formation of amyloid-β (Aβ) fibrils is a central pathogenic feature of Alzheimer's disease. Cell-secreted extracellular vesicles (EVs) have been suggested as disease modulators, although their exact roles and relations to Aβ pathology remain unclear. We combined kinetics assays and biophysical analyses to explore how small (<220 nm) EVs from neuronal and non-neuronal human cell lines affected the aggregation of the disease-associated Aβ variant Aβ(1-42) into amyloid fibrils. Using thioflavin-T monitored kinetics and seeding assays, we found that EVs reduced Aβ(1-42) aggregation by inhibiting fibril elongation. Morphological analyses revealed this to result in the formation of short fibril fragments with increased thicknesses and less apparent twists. We suggest that EVs may have protective roles by reducing Aβ(1-42) amyloid loads, but also note that the formation of small amyloid fragments could be problematic from a neurotoxicity perspective. EVs may therefore have double-edged roles in the regulation of Aβ pathology in Alzheimer's disease.
Keywords: Alzheimer’s disease; EVs; amyloid kinetics; amyloid-β; extracellular vesicles; protein aggregation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

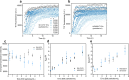
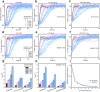

Comment in
-
Comment on "Extracellular Vesicles Slow Down Aβ(1-42) Aggregation by Interfering with the Amyloid Fibril Elongation Step".ACS Chem Neurosci. 2024 Nov 6;15(21):3791-3793. doi: 10.1021/acschemneuro.4c00601. Epub 2024 Oct 9. ACS Chem Neurosci. 2024. PMID: 39382326 Free PMC article.
References
-
- McLean C. A.; Cherny R. A.; Fraser F. W.; Fuller S. J.; Smith M. J.; Beyreuther K.; Bush A. I.; Masters C. L. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann. Neurol. 1999, 46 (6), 860–866. 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

