Pilot RCT comparing low-dose naltrexone, gabapentin and placebo to reduce pain among people with HIV with alcohol problems
- PMID: 38408060
- PMCID: PMC10896547
- DOI: 10.1371/journal.pone.0297948
Pilot RCT comparing low-dose naltrexone, gabapentin and placebo to reduce pain among people with HIV with alcohol problems
Abstract
Background: To estimate the effects on pain of two medications (low-dose naltrexone and gabapentin) compared to placebo among people with HIV (PWH) with heavy alcohol use and chronic pain.
Methods: We conducted a pilot, randomized, double-blinded, 3-arm study of PWH with chronic pain and past-year heavy alcohol use in 2021. Participants were recruited in St. Petersburg, Russia, and randomized to receive daily low-dose naltrexone (4.5mg), gabapentin (up to 1800mg), or placebo. The two primary outcomes were change in self-reported pain severity and pain interference measured with the Brief Pain Inventory from baseline to 8 weeks.
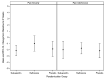
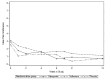
Results: Participants (N = 45, 15 in each arm) had the following baseline characteristics: 64% male; age 41 years (SD±7); mean 2 (SD±4) heavy drinking days in the past month and mean pain severity and interference were 3.2 (SD±1) and 3.0 (SD±2), respectively. Pain severity decreased for all three arms. Mean differences in change in pain severity for gabapentin vs. placebo, and naltrexone vs. placebo were -0.27 (95% confidence interval [CI] -1.76, 1.23; p = 0.73) and 0.88 (95% CI -0.7, 2.46; p = 0.55), respectively. Pain interference decreased for all three arms. Mean differences in change in pain interference for gabapentin vs. placebo, and naltrexone vs. placebo was 0.16 (95% CI -1.38, 1.71; p = 0.83) and 0.40 (95% CI -1.18, 1.99; p = 0.83), respectively.
Conclusion: Neither gabapentin nor low-dose naltrexone appeared to improve pain more than placebo among PWH with chronic pain and past-year heavy alcohol use.
Clinical trial registration: ClinicalTrials.gov (NCT4052139).
Trial registration: ClinicalTrials.gov NCT04052139.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
Employees of the funder NIH (K. Bryant and M. Backonja) are co-authors on this manuscript and helped guide analysis, interpretation and presentation of data and participated in the decision to submit the manuscript for publication. The funder provided support in the form of salaries for authors [K. Bryant, M. Backonja], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section. D. Cheng serves on Data Safety Monitoring Boards for Janssen Research & Development. This does not alter our adherence to PLOS ONE policies on sharing data and materials. The remaining authors report no conflicts of interest.
Figures




References
-
- Breivik H, Eisenberg E, O’Brien T, OPENMinds. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health. 2013. Dec 24;13:1229. doi: 10.1186/1471-2458-13-1229 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

