Nutraceuticals known to promote hair growth do not interfere with the inhibitory action of tamoxifen in MCF7, T47D and BT483 breast cancer cell lines
- PMID: 38408073
- PMCID: PMC10896530
- DOI: 10.1371/journal.pone.0297080
Nutraceuticals known to promote hair growth do not interfere with the inhibitory action of tamoxifen in MCF7, T47D and BT483 breast cancer cell lines
Abstract
Background: Hair loss/thinning is a common side effect of tamoxifen in estrogen receptor (ER) positive breast cancer therapy. Some nutraceuticals known to promote hair growth are avoided during breast cancer therapy for fear of phytoestrogenic activity. However, not all botanical ingredients have similarities to estrogens, and in fact, no information exists as to the true interaction of these ingredients with tamoxifen. Therefore, this study sought to ascertain the effect of nutraceuticals (+/- estrogen/tamoxifen), on proliferation of breast cancer cells and the relative expression of ERα/β.
Methods: Kelp, Astaxanthin, Saw Palmetto, Tocotrienols, Maca, Horsetail, Resveratrol, Curcumin and Ashwagandha were assessed on proliferation of MCF7, T47D and BT483 breast cancer cell lines +/- 17β-estradiol and tamoxifen. Each extract was analysed by high performance liquid chromatography (HPLC) prior to use. Cellular ERα and ERβ expression was assessed by qRT-PCR and western blot. Changes in the cellular localisation of ERα:ERβ and their ratio following incubation with the nutraceuticals was confirmed by immunocytochemistry.
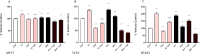
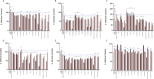
Results: Estradiol stimulated DNA synthesis in three different breast cancer cell lines: MCF7, T47D and BT483, which was inhibited by tamoxifen; this was mirrored by a specific ERa agonist in T47D and BT483 cells. Overall, nutraceuticals did not interfere with tamoxifen inhibition of estrogen; some even induced further inhibition when combined with tamoxifen. The ERα:ERβ ratio was higher at mRNA and protein level in all cell lines. However, incubation with nutraceuticals induced a shift to higher ERβ expression and a localization of ERs around the nuclear periphery.
Conclusions: As ERα is the key driver of estrogen-dependent breast cancer, if nutraceuticals have a higher affinity for ERβ they may offer a protective effect, particularly if they synergize and augment the actions of tamoxifen. Since ERβ is the predominant ER in the hair follicle, further studies confirming whether nutraceuticals can shift the ratio towards ERβ in hair follicle cells would support a role for them in hair growth. Although more research is needed to assess safety and efficacy, this promising data suggests the potential of nutraceuticals as adjuvant therapy for hair loss in breast cancer patients receiving endocrine therapy.
Copyright: © 2024 Baker et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
This study was supported by Nutraceutical Wellness, Inc. New York, USA This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
-
- Hartman J, Lindberg K, Morani A, Inzunza J, Strom A, Gustafsson JA. Estrogen Receptor β Inhibits Angiogenesis and Growth of T47D Breast Cancer Xenografts. Cancer Res.2006. Dec; 66 (23): 11207–11213 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

