Circadian regulation of sinoatrial nodal cell pacemaking function: Dissecting the roles of autonomic control, body temperature, and local circadian rhythmicity
- PMID: 38408116
- PMCID: PMC10927146
- DOI: 10.1371/journal.pcbi.1011907
Circadian regulation of sinoatrial nodal cell pacemaking function: Dissecting the roles of autonomic control, body temperature, and local circadian rhythmicity
Abstract
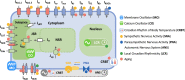
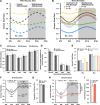
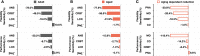
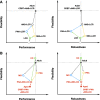
Strong circadian (~24h) rhythms in heart rate (HR) are critical for flexible regulation of cardiac pacemaking function throughout the day. While this circadian flexibility in HR is sustained in diverse conditions, it declines with age, accompanied by reduced maximal HR performance. The intricate regulation of circadian HR involves the orchestration of the autonomic nervous system (ANS), circadian rhythms of body temperature (CRBT), and local circadian rhythmicity (LCR), which has not been fully understood. Here, we developed a mathematical model describing ANS, CRBT, and LCR in sinoatrial nodal cells (SANC) that accurately captures distinct circadian patterns in adult and aged mice. Our model underscores how the alliance among ANS, CRBT, and LCR achieves circadian flexibility to cover a wide range of firing rates in SANC, performance to achieve maximal firing rates, while preserving robustness to generate rhythmic firing patterns irrespective of external conditions. Specifically, while ANS dominates in promoting SANC flexibility and performance, CRBT and LCR act as primary and secondary boosters, respectively, to further enhance SANC flexibility and performance. Disruption of this alliance with age results in impaired SANC flexibility and performance, but not robustness. This unexpected outcome is primarily attributed to the age-related reduction in parasympathetic activities, which maintains SANC robustness while compromising flexibility. Our work sheds light on the critical alliance of ANS, CRBT, and LCR in regulating time-of-day cardiac pacemaking function and dysfunction, offering insights into novel therapeutic targets for the prevention and treatment of cardiac arrhythmias.
Copyright: © 2024 Li, Kim. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
cAMP-Dependent Signaling Restores AP Firing in Dormant SA Node Cells via Enhancement of Surface Membrane Currents and Calcium Coupling.Front Physiol. 2021 Apr 9;12:596832. doi: 10.3389/fphys.2021.596832. eCollection 2021. Front Physiol. 2021. PMID: 33897445 Free PMC article.
-
Spontaneous, local diastolic subsarcolemmal calcium releases in single, isolated guinea-pig sinoatrial nodal cells.PLoS One. 2017 Sep 25;12(9):e0185222. doi: 10.1371/journal.pone.0185222. eCollection 2017. PLoS One. 2017. PMID: 28945810 Free PMC article.
-
Unique Ca2+-Cycling Protein Abundance and Regulation Sustains Local Ca2+ Releases and Spontaneous Firing of Rabbit Sinoatrial Node Cells.Int J Mol Sci. 2018 Jul 25;19(8):2173. doi: 10.3390/ijms19082173. Int J Mol Sci. 2018. PMID: 30044420 Free PMC article. Review.
-
CaMKII-dependent phosphorylation regulates basal cardiac pacemaker function via modulation of local Ca2+ releases.Am J Physiol Heart Circ Physiol. 2016 Sep 1;311(3):H532-44. doi: 10.1152/ajpheart.00765.2015. Epub 2016 Jul 8. Am J Physiol Heart Circ Physiol. 2016. PMID: 27402669 Free PMC article.
-
A Tangled Threesome: Circadian Rhythm, Body Temperature Variations, and the Immune System.Biology (Basel). 2021 Jan 18;10(1):65. doi: 10.3390/biology10010065. Biology (Basel). 2021. PMID: 33477463 Free PMC article. Review.
Cited by
-
Circadian influences on sudden cardiac death and cardiac electrophysiology.J Mol Cell Cardiol. 2025 Mar;200:93-112. doi: 10.1016/j.yjmcc.2025.01.006. Epub 2025 Jan 27. J Mol Cell Cardiol. 2025. PMID: 39864795 Review.
-
Circadian Rhythms, Immune Regulation, and the Risk for Sepsis: Circadian Rhythms and Neonatal Care.Clin Perinatol. 2025 Mar;52(1):185-197. doi: 10.1016/j.clp.2024.10.012. Epub 2024 Nov 26. Clin Perinatol. 2025. PMID: 39892952 Review.
-
Cardiogenic and chronobiological mechanisms in seizure-induced sinus arrhythmias.PLoS Comput Biol. 2025 Jul 16;21(7):e1013318. doi: 10.1371/journal.pcbi.1013318. eCollection 2025 Jul. PLoS Comput Biol. 2025. PMID: 40668822 Free PMC article.
References
-
- Alibhai FJ, Tsimakouridze EV, Reitz CJ, Pyle WG, Martino TA. The Cardiac Clock. In: Gumz ML, editor. Circadian Clocks: Role in Health and Disease. New York, NY: Springer New York; 2016. p. 225–50.
MeSH terms
LinkOut - more resources
Full Text Sources

