Stabilizing Monoatomic Two-Coordinate Bismuth(I) and Bismuth(II) Using a Redox Noninnocent Bis(germylene) Ligand
- PMID: 38408197
- PMCID: PMC10921399
- DOI: 10.1021/jacs.3c13016
Stabilizing Monoatomic Two-Coordinate Bismuth(I) and Bismuth(II) Using a Redox Noninnocent Bis(germylene) Ligand
Abstract
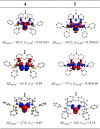
The formation of isolable monatomic BiI complexes and BiII radical species is challenging due to the pronounced reducing nature of metallic bismuth. Here, we report a convenient strategy to tame BiI and BiII atoms by taking advantage of the redox noninnocent character of a new chelating bis(germylene) ligand. The remarkably stable novel BiI cation complex 4, supported by the new bis(iminophosphonamido-germylene)xanthene ligand [(P)GeII(Xant)GeII(P)] 1, [(P)GeII(Xant)GeII(P) = Ph2P(NtBu)2GeII(Xant)GeII(NtBu)2PPh2, Xant = 9,9-dimethyl-xanthene-4,5-diyl], was synthesized by a two-electron reduction of the cationic BiIIII2 precursor complex 3 with cobaltocene (Cp2Co) in a molar ratio of 1:2. Notably, owing to the redox noninnocent character of the germylene moieties, the positive charge of BiI cation 4 migrates to one of the Ge atoms in the bis(germylene) ligand, giving rise to a germylium(germylene) BiI complex as suggested by DFT calculations and X-ray photoelectron spectroscopy (XPS). Likewise, migration of the positive charge of the BiIIII2 cation of 3 results in a bis(germylium)BiIIII2 complex. The delocalization of the positive charge in the ligand engenders a much higher stability of the BiI cation 4 in comparison to an isoelectronic two-coordinate Pb0 analogue (plumbylone; decomposition below -30 °C). Interestingly, 4[BArF] undergoes a reversible single-electron transfer (SET) reaction (oxidation) to afford the isolable BiII radical complex 5 in 5[BArF]2. According to electron paramagnetic resonance (EPR) spectroscopy, the unpaired electron predominantly resides at the BiII atom. Extending the redox reactivity of 4[OTf] employing AgOTf and MeOTf affords BiIII(OTf)2 complex 7 and BiIIIMe complex 8, respectively, demonstrating the high nucleophilic character of BiI cation 4.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
















References
-
- Yadav S.; Saha S.; Sen S. S. Compounds with Low-Valent p-Block Elements for Small Molecule Activation and Catalysis. ChemCatChem 2016, 8, 486–501. 10.1002/cctc.201501015. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

