KIT ATP-Binding Pocket/Activation Loop Mutations in GI Stromal Tumor: Emerging Mechanisms of Kinase Inhibitor Escape
- PMID: 38408285
- PMCID: PMC11095889
- DOI: 10.1200/JCO.23.01197
KIT ATP-Binding Pocket/Activation Loop Mutations in GI Stromal Tumor: Emerging Mechanisms of Kinase Inhibitor Escape
Abstract
Purpose: Imatinib resistance in GI stromal tumors (GISTs) is primarily caused by secondary KIT mutations, and clonal heterogeneity of these secondary mutations represents a major treatment obstacle. KIT inhibitors used after imatinib have clinical activity, albeit with limited benefit. Ripretinib is a potent inhibitor of secondary KIT mutations in the activation loop (AL). However, clinical benefit in fourth line remains limited and the molecular mechanisms of ripretinib resistance are largely unknown.
Patients and methods: Progressing lesions of 25 patients with GISTs refractory to ripretinib were sequenced for KIT resistance mutations. Resistant genotypes were validated and characterized using novel cell line models and in silico modeling.
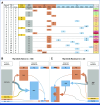
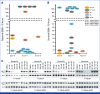
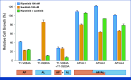
Results: GISTs progressing on ripretinib were enriched for secondary mutations in the ATP-binding pocket (AP), which frequently occur in cis with preexisting AL mutations, resulting in highly resistant AP/AL genotypes. AP/AL mutations were rarely observed in a cohort of progressing GIST samples from the preripretinib era but represented 50% of secondary KIT mutations in patients with tumors resistant to ripretinib. In GIST cell lines harboring secondary KIT AL mutations, the sole genomic escape mechanisms during ripretinib drug selection were AP/AL mutations. Ripretinib and sunitinib synergize against mixed clones with secondary AP or AL mutants but do not suppress clones with AP/AL genotypes.
Conclusion: Our findings underscore that KIT remains the central oncogenic driver even in late lines of GIST therapy. KIT-inhibitor combinations may suppress resistance because of secondary KIT mutations. However, the emergence of KIT AP/AL mutations after ripretinib treatment calls for new strategies in the development of next-generation KIT inhibitors.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures



Comment in
-
Comprehensive Discussions on KIT ATP-Binding Pocket/Activation Loop Mutations in Ripretinib Resistance.J Clin Oncol. 2024 Sep 20;42(27):3259. doi: 10.1200/JCO.24.00673. Epub 2024 Jul 8. J Clin Oncol. 2024. PMID: 38976817 No abstract available.
References
-
- Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—A population-based study in western Sweden. Cancer. 2005;103:821–829. - PubMed
-
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

