Synthesis and Deconstruction of Polyethylene-type Materials
- PMID: 38408312
- PMCID: PMC10941192
- DOI: 10.1021/acs.chemrev.3c00587
Synthesis and Deconstruction of Polyethylene-type Materials
Abstract
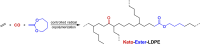
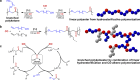
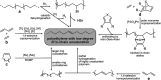
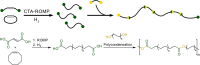
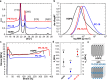
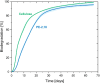
Polyethylene deconstruction to reusable smaller molecules is hindered by the chemical inertness of its hydrocarbon chains. Pyrolysis and related approaches commonly require high temperatures, are energy-intensive, and yield mixtures of multiple classes of compounds. Selective cleavage reactions under mild conditions (<ca. 200 °C) are key to improve the efficacy of chemical recycling and upcycling approaches. These can be enabled by introduction of low densities of predetermined breaking points in the polyethylene chains during the step-growth or chain-growth synthetic construction of designed-for-recycling polyethylene-type materials. Alternatively, they can be accomplished by postpolymerization functionalization of postconsumer polyethylene waste via dehydrogenation and follow-up reactions or through oxidation to long-chain dicarboxylates. Deconstruction of litter under environmental conditions via the aforementioned break points can alleviate plastics' persistency, as a backstop to closed-loop recycling.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











References
-
- Policy Scenarios to 2060. Global Plastics Outlook ;OECD, 2022. 10.1787/aa1edf33-en - DOI
-
- Plastics - the Facts 2020. Plastics Europe. Online at https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/ (accessed 2023-10-28).
-
- Vollmer I.; Jenks M. J. F.; Roelands M. C. P.; White R. J.; van Harmelen T.; de Wild P.; van der Laan G. P.; Meirer F.; Keurentjes J. T. F.; Weckhuysen B. M. Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew. Chem., Int. Ed. 2020, 59, 15402–15423. 10.1002/anie.201915651. - DOI - PMC - PubMed
-
- Vogt B. D.; Stokes K. K.; Kumar S. K. Why is Recycling of Postconsumer Plastics so Challenging?. ACS Appl. Polym. Mater. 2021, 3, 4325–4346. 10.1021/acsapm.1c00648. - DOI
Publication types
LinkOut - more resources
Full Text Sources

