Revealing the Intrinsic Restructuring of Bi2O3 Nanoparticles into Bi Nanosheets during Electrochemical CO2 Reduction
- PMID: 38408369
- PMCID: PMC10921375
- DOI: 10.1021/acsami.3c18285
Revealing the Intrinsic Restructuring of Bi2O3 Nanoparticles into Bi Nanosheets during Electrochemical CO2 Reduction
Abstract
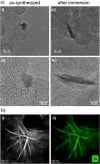
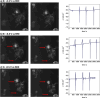
Bismuth is a catalyst material that selectively produces formate during the electrochemical reduction of CO2. While different synthesis strategies have been employed to create electrocatalysts with better performance, the restructuring of bismuth precatalysts during the reaction has also been previously reported. The mechanism behind the change has, however, remained unclear. Here, we show that Bi2O3 nanoparticles supported on Vulcan carbon intrinsically transform into stellated nanosheet aggregates upon exposure to an electrolyte. Liquid cell transmission electron microscopy observations first revealed the gradual restructuring of the nanoparticles into nanosheets in the presence of 0.1 M KHCO3 without an applied potential. Our experiments also associated the restructuring with solubility of bismuth in the electrolyte. While the consequent agglomerates were stable under moderate negative potentials (-0.3 VRHE), they dissolved over time at larger negative potentials (-0.4 and -0.5 VRHE). Operando Raman spectra collected during the reaction showed that under an applied potential, the oxide particles reduced to metallic bismuth, thereby confirming the metal as the working phase for producing formate. These results inform us about the working morphology of these electrocatalysts and their formation and degradation mechanisms.
Keywords: bismuth oxide nanoparticles; carbon dioxide electroreduction; catalyst restructuring; in situ studies; liquid cell transmission electron microscopy; operando Raman spectroscopy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Bismuth Nanosheets Derived by In Situ Morphology Transformation of Bismuth Oxides for Selective Electrochemical CO2 Reduction to Formate.ACS Appl Mater Interfaces. 2022 Mar 30;14(12):14210-14217. doi: 10.1021/acsami.1c25217. Epub 2022 Mar 17. ACS Appl Mater Interfaces. 2022. PMID: 35297598
-
Porous Bi Nanosheets Derived from β-Bi2O3 for Efficient Electrocatalytic CO2 Reduction to Formate.ACS Appl Mater Interfaces. 2024 Aug 14;16(32):42109-42117. doi: 10.1021/acsami.4c05842. Epub 2024 Aug 1. ACS Appl Mater Interfaces. 2024. PMID: 39088819
-
Monitoring chalcogenide ions-guided in situ transform active sites of tailored bismuth electrocatalysts for CO2 reduction to formate.Proc Natl Acad Sci U S A. 2025 Mar 11;122(10):e2420922122. doi: 10.1073/pnas.2420922122. Epub 2025 Mar 5. Proc Natl Acad Sci U S A. 2025. PMID: 40042908
-
Decorating graphdiyne on ultrathin bismuth subcarbonate nanosheets to promote CO2 electroreduction to formate.Sci Bull (Beijing). 2021 Aug 15;66(15):1533-1541. doi: 10.1016/j.scib.2021.03.020. Epub 2021 Mar 23. Sci Bull (Beijing). 2021. PMID: 36654282
-
Engineering Under-Coordinated Active Sites with Tailored Chemical Microenvironments over Mosaic Bismuth Nanosheets for Selective CO2 Electroreduction to Formate.Small. 2023 Apr;19(16):e2207305. doi: 10.1002/smll.202207305. Epub 2023 Jan 20. Small. 2023. PMID: 36670091
Cited by
-
Recent Developments in Single-Entity Electrochemistry.Anal Chem. 2024 May 21;96(20):8036-8055. doi: 10.1021/acs.analchem.4c01406. Epub 2024 May 10. Anal Chem. 2024. PMID: 38727715 Free PMC article. Review. No abstract available.
-
Unveiling the Origin of pH-Dependent Catalytic Performance of Bi2O3 Nanostructure for Electrochemical CO2 Reduction.J Phys Chem Lett. 2025 Apr 17;16(15):3761-3768. doi: 10.1021/acs.jpclett.5c00103. Epub 2025 Apr 7. J Phys Chem Lett. 2025. PMID: 40193254 Free PMC article.
References
-
- Hori Y. Utilization of Carbon Dioxide by Electrolytic Reduction. Sen’i Gakkaishi 1992, 48 (1), 38–42. 10.2115/fiber.48.P38. - DOI
-
- Alper E.; Yuksel Orhan O. CO2 Utilization: Developments in Conversion Processes. Petroleum 2017, 3 (1), 109–126. 10.1016/j.petlm.2016.11.003. - DOI
-
- Osman A. I.; Hefny M.; Abdel Maksoud M. I. A.; Elgarahy A. M.; Rooney D. W. Recent Advances in Carbon Capture Storage and Utilisation Technologies: A Review. Environ. Chem. Lett. 2021, 19 (2), 797–849. 10.1007/s10311-020-01133-3. - DOI
-
- Hurd C. L.; Lenton A.; Tilbrook B.; Boyd P. W. Current Understanding and Challenges for Oceans in a Higher-CO2 World. Nature Climate Change. 2018, 8, 686–694. 10.1038/s41558-018-0211-0. - DOI
-
- Jacobson M. Z. The Health and Climate Impacts of Carbon Capture and Direct Air Capture. Energy Environ. Sci. 2019, 12 (12), 3567–3574. 10.1039/C9EE02709B. - DOI
LinkOut - more resources
Full Text Sources

