Reframing sepsis immunobiology for translation: towards informative subtyping and targeted immunomodulatory therapies
- PMID: 38408467
- PMCID: PMC11025021
- DOI: 10.1016/S2213-2600(23)00468-X
Reframing sepsis immunobiology for translation: towards informative subtyping and targeted immunomodulatory therapies
Abstract
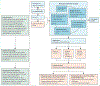
Sepsis is a common and deadly condition. Within the current model of sepsis immunobiology, the framing of dysregulated host immune responses into proinflammatory and immunosuppressive responses for the testing of novel treatments has not resulted in successful immunomodulatory therapies. Thus, the recent focus has been to parse observable heterogeneity into subtypes of sepsis to enable personalised immunomodulation. In this Personal View, we highlight that many fundamental immunological concepts such as resistance, disease tolerance, resilience, resolution, and repair are not incorporated into the current sepsis immunobiology model. The focus for addressing heterogeneity in sepsis should be broadened beyond subtyping to encompass the identification of deterministic molecular networks or dominant mechanisms. We explicitly reframe the dysregulated host immune responses in sepsis as altered homoeostasis with pathological disruption of immune-driven resistance, disease tolerance, resilience, and resolution mechanisms. Our proposal highlights opportunities to identify novel treatment targets and could enable successful immunomodulation in the future.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MS-H is supported by the UK National Institute for Health and Care Research (NIHR) Clinician Scientist Award (CS-2016-16-011; 2017–2023). MPS's laboratory is supported by the Calouste Gulbenkian Foundation, La Caixa Foundation (HR18-00502), Fundação para a Ciência e a Tecnologia (GlucoInfect, 5723/2014; FEDER029411, FEDER/29411/2017; Infectenergy, PTDC/MED-FSL/4681/2020; MalBil, 2022.02426.PTDC), the Oeiras–European Research Council Frontier Research Incentive Awards, SymbNET Research Grants (H2020-WIDESPREAD-2020-5-952537), and Congento (LISBOA-01-0145-FEDER-022170). MPS is an associate member of the Deutsche Forschungsgemeinschaft (DFG) Balance of the Microverse initiative (EXC 2051; 390713860). MB is principal investigator of the DFG Balance of the Microverse initiative (EXC 2051; 390713860). JCK is supported by the Medical Research Council (MR/V002503/1), a Wellcome Trust Investigator Award (204969/Z/16/Z), and Wellcome Trust grants (090532/Z/09/Z and 203141/Z/16/Z) to the Wellcome Centre for Human Genetics, Nuffield Department of Medicine, University of Oxford, and the NIHR Oxford Biomedical Research Centre. JM reports grants from the Canadian Institutes of Health Research and has served as chair of data and safety monitoring boards for AM Pharma and Adrenomed. CWS reports grants from the US National Institutes of Health National Institute of General Medical Sciences, during the conduct of the study, and personal fees from Inotrem and Beckman Coulter, outside of the submitted work. IBM has received funding or consultancy fees from Abbvie, Amgen, Bristol Myers Squibb, Cabaletta, Causeway, Eli Lilly, Evelo, GSK, Janssen, Moonlake, Novartis, Pfizer, and UCB. TvdP has received grants from the EU's Horizon 2020 research and innovation funding programme (grant agreements: Flagellin Aerosol Therapy in Treatment of Drug-resistant Bacterial Pneumonia, 847786; ImmunoSEP, 847422). All other authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

