Impact of high-fat diet on cognitive behavior and central and systemic inflammation with aging and sex differences in mice
- PMID: 38408498
- PMCID: PMC11019935
- DOI: 10.1016/j.bbi.2024.02.025
Impact of high-fat diet on cognitive behavior and central and systemic inflammation with aging and sex differences in mice
Abstract
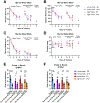
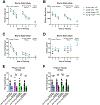
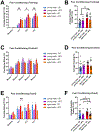
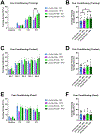
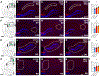
Aging and age-related diseases are associated with cellular stress, metabolic imbalance, oxidative stress, and neuroinflammation, accompanied by cognitive impairment. Lifestyle factors such as diet, sleep fragmentation, and stress can potentiate damaging cellular cascades and lead to an acceleration of brain aging and cognitive impairment. High-fat diet (HFD) has been associated with obesity, metabolic disorders like diabetes, and cardiovascular disease. HFD also induces neuroinflammation, impairs learning and memory, and may increase anxiety-like behavior. Effects of a HFD may also vary between sexes. The interaction between Age- and Sex- and Diet-related changes in neuroinflammation and cognitive function is an important and poorly understood area of research. This study was designed to examine the effects of HFD on neuroinflammation, behavior, and neurodegeneration in mice in the context of aging or sex differences. In a series of studies, young (2-3 months) or old (12-13 months) C57BL/6J male mice or young male and female C57Bl/6J mice were fed either a standard diet (SD) or a HFD for 5-6 months. Behavior was assessed in Activity Chamber, Y-maze, Novel Place Recognition, Novel Object Recognition, Elevated Plus Maze, Open Field, Morris Water Maze, and Fear Conditioning. Post-mortem analyses assessed a panel of inflammatory markers in the plasma and hippocampus. Additionally, proteomic analysis of the hypothalamus, neurodegeneration, neuroinflammation in the locus coeruleus, and neuroinflammation in the hippocampus were assessed in a subset of young and aged male mice. We show that HFD increased body weight and decreased locomotor activity across groups compared to control mice fed a SD. HFD altered anxiety-related exploratory behavior. HFD impaired spatial learning and recall in young male mice and impaired recall in cued fear conditioning in young and aged male mice, with no effects on spatial learning or fear conditioning in young female mice. Effects of Age and Sex were observed on neuroinflammatory cytokines, with only limited effects of HFD. HFD had a more significant impact on systemic inflammation in plasma across age and sex. Aged male mice had induction of microglial immunoreactivity in both the locus coeruleus (LC) and hippocampus an effect that HFD exacerbated in the hippocampal CA1 region. Proteomic analysis of the hypothalamus revealed changes in pathways related to metabolism and neurodegeneration with both aging and HFD in male mice. Our findings suggest that HFD induces widespread systemic inflammation and limited neuroinflammation. In addition, HFD alters exploratory behavior in male and female mice, and impairs learning and memory in male mice. These results provide valuable insight into the impact of diet on cognition and aging pathophysiology.
Keywords: Aging; Behavior; Chemokines; Cytokines; Growth factors; High-fat diet; Inflammation; Learning and memory; Locus coeruleus; Neuroinflammation; Sex differences.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

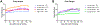







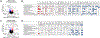
Similar articles
-
Age-related neuroinflammation and pathology in the locus coeruleus and hippocampus: beta-adrenergic antagonists exacerbate impairment of learning and memory in aged mice.Neurobiol Aging. 2021 Oct;106:241-256. doi: 10.1016/j.neurobiolaging.2021.06.012. Epub 2021 Jun 20. Neurobiol Aging. 2021. PMID: 34320462 Free PMC article.
-
Young adult and aged female rats are vulnerable to amygdala-dependent, but not hippocampus-dependent, memory impairment following short-term high-fat diet.Brain Res Bull. 2023 Apr;195:145-156. doi: 10.1016/j.brainresbull.2023.03.001. Epub 2023 Mar 2. Brain Res Bull. 2023. PMID: 36870621 Free PMC article. Review.
-
Behavioral changes in male mice fed a high-fat diet are associated with IL-1β expression in specific brain regions.Physiol Behav. 2017 Feb 1;169:130-140. doi: 10.1016/j.physbeh.2016.11.016. Epub 2016 Nov 19. Physiol Behav. 2017. PMID: 27876639
-
High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory.Neurobiol Aging. 2017 Oct;58:88-101. doi: 10.1016/j.neurobiolaging.2017.06.014. Epub 2017 Jun 24. Neurobiol Aging. 2017. PMID: 28719855 Free PMC article.
-
High-fat diet and neuroinflammation: The role of mitochondria.Pharmacol Res. 2025 Feb;212:107615. doi: 10.1016/j.phrs.2025.107615. Epub 2025 Jan 20. Pharmacol Res. 2025. PMID: 39842474 Review.
Cited by
-
Altered cognitive function in obese patients: relationship to gut flora.Mol Cell Biochem. 2025 Jun;480(6):3553-3567. doi: 10.1007/s11010-024-05201-y. Epub 2025 Feb 12. Mol Cell Biochem. 2025. PMID: 39937394 Free PMC article. Review.
-
Inflammaging: The Next Challenge-Exploring the Role of Gut Microbiota, Environmental Factors, and Sex Differences.Biomedicines. 2024 Aug 1;12(8):1716. doi: 10.3390/biomedicines12081716. Biomedicines. 2024. PMID: 39200181 Free PMC article. Review.
-
High-fat and High-sucrose Diet-induced Hypothalamic Inflammation Shows Sex Specific Features in Mice.Neurochem Res. 2024 Dec;49(12):3356-3366. doi: 10.1007/s11064-024-04243-4. Epub 2024 Sep 20. Neurochem Res. 2024. PMID: 39302596 Free PMC article.
-
Sex differences in the outcomes of modifiable lifestyle factors for cognitive aging: neuroinflammation and microglia as key underlying mechanisms.Front Aging Neurosci. 2025 Jul 22;17:1642043. doi: 10.3389/fnagi.2025.1642043. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40766178 Free PMC article. Review.
-
High-fat diet-induced L-saccharopine accumulation inhibits estradiol synthesis and damages oocyte quality by disturbing mitochondrial homeostasis.Gut Microbes. 2024 Jan-Dec;16(1):2412381. doi: 10.1080/19490976.2024.2412381. Epub 2024 Oct 16. Gut Microbes. 2024. PMID: 39410876 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

