Depression follow-up monitoring with the PHQ-9: an open cluster-randomised controlled trial
- PMID: 38408790
- PMCID: PMC11221421
- DOI: 10.3399/BJGP.2023.0539
Depression follow-up monitoring with the PHQ-9: an open cluster-randomised controlled trial
Abstract
Background: Outcome monitoring of depression treatment is recommended but there is a lack of evidence on patient benefit in primary care.
Aim: To test monitoring depression using the Patient Health Questionnaire (PHQ-9) with patient feedback.
Design and setting: An open cluster-randomised controlled trial was undertaken in 141 group practices.
Method: Adults with new depressive episodes were recruited through record searches and opportunistically. The exclusion criteria were as follows: dementia; psychosis; substance misuse; and suicide risk. The PHQ-9 was administered soon after diagnosis, and 10-35 days later. The primary outcome was the Beck Depression Inventory (BDI-II) score at 12 weeks. The secondary outcomes were as follows: BDI-II at 26 weeks; Work and Social Adjustment Scale (WSAS) and EuroQol EQ-5D-5L quality of life at 12 and 26 weeks; antidepressant treatment; mental health and social service contacts; adverse events, and Medical Interview Satisfaction Scale (MISS) over 26 weeks.
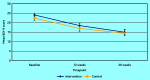
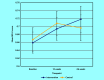
Results: In total, 302 patients were recruited to the intervention arm and 227 to the controls. At 12 weeks, 254 (84.1%) and 199 (87.7%) were followed-up, respectively. Only 40.9% of patients in the intervention had a GP follow-up PHQ-9 recorded. There was no significant difference in BDI-II score at 12 weeks (mean difference -0.46; 95% confidence interval [CI] = -2.16 to 1.26; adjusted for baseline depression, baseline anxiety, sociodemographic factors, and clustering by practice). EQ-5D-5L quality-of-life scores were higher in the intervention arm at 26 weeks (adjusted mean difference 0.053; 95% CI = 0.013 to 0.093. A clinically significant difference in depression at 26 weeks could not be ruled out. No significant differences were found in social functioning, adverse events, or satisfaction. In a per-protocol analysis, antidepressant use and mental health contacts were significantly greater in patients in the intervention arm with a recorded follow-up PHQ-9 (P = 0.025 and P = 0.010, respectively).
Conclusion: No evidence was found of improved depression outcome at 12 weeks from monitoring. The findings of possible benefits over 26 weeks warrant replication, investigating possible mechanisms, preferably with automated delivery of monitoring and more instructive feedback.
Keywords: depression; mental health; mood disorders; patient reported outcome measures; primary health care.
© The Authors.
Conflict of interest statement
All authors completed a disclosure form at:
Figures
Comment in
-
Depression follow-up monitoring with the PHQ-9: open cluster-randomised controlled trial.Br J Gen Pract. 2024 Apr 25;74(742):202-203. doi: 10.3399/bjgp24X737109. Print 2024 May. Br J Gen Pract. 2024. PMID: 38664056 Free PMC article. No abstract available.
Similar articles
-
Patient-reported outcome measures for monitoring primary care patients with depression: the PROMDEP cluster RCT and economic evaluation.Health Technol Assess. 2024 Mar;28(17):1-95. doi: 10.3310/PLRQ4216. Health Technol Assess. 2024. PMID: 38551155 Free PMC article. Clinical Trial.
-
Patient-reported outcome measures for monitoring primary care patients with depression (PROMDEP): study protocol for a randomised controlled trial.Trials. 2020 May 29;21(1):441. doi: 10.1186/s13063-020-04344-9. Trials. 2020. PMID: 32471492 Free PMC article.
-
Patient-reported outcome measures for monitoring primary care patients with depression: PROMDEP feasibility randomised trial.BMJ Open. 2017 Mar 30;7(3):e015266. doi: 10.1136/bmjopen-2016-015266. BMJ Open. 2017. PMID: 28363932 Free PMC article. Clinical Trial.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
Pharmacological interventions for treatment-resistant depression in adults.Cochrane Database Syst Rev. 2019 Dec 17;12(12):CD010557. doi: 10.1002/14651858.CD010557.pub2. Cochrane Database Syst Rev. 2019. PMID: 31846068 Free PMC article.
Cited by
-
Depression follow-up monitoring with the PHQ-9: open cluster-randomised controlled trial.Br J Gen Pract. 2024 Apr 25;74(742):202-203. doi: 10.3399/bjgp24X737109. Print 2024 May. Br J Gen Pract. 2024. PMID: 38664056 Free PMC article. No abstract available.
-
Interventions to improve mental help-seeking behaviours in individuals with depressive symptoms: a systematic review and meta-analysis.BMC Psychiatry. 2025 May 23;25(1):528. doi: 10.1186/s12888-025-06976-0. BMC Psychiatry. 2025. PMID: 40410702 Free PMC article.
-
Effect of General Anesthesia Combined with Transversus Abdominis Plane Block on Postoperative Sleep Disorders in Elderly Patients Undergoing Gastrointestinal Tumor Surgery: A Prospective, Randomized Controlled Trial.Nat Sci Sleep. 2025 Jan 7;17:17-25. doi: 10.2147/NSS.S486711. eCollection 2025. Nat Sci Sleep. 2025. PMID: 39801627 Free PMC article. Clinical Trial.
References
-
- National Institute for Health and Care Excellence (NICE) Depression in adults: treatment and management. NICE guideline [NG222] 2022. https://www.nice.org.uk/guidance/ng222/chapter/Recommendations#recogniti... (accessed 20 March 2024). - PubMed
-
- Health Resources and Services Administration Uniform Data System Clinical Quality Measures. 2020 https://bphc.hrsa.gov/sites/default/files/bphc/data-reporting/2020-clini... (accessed 20 March 2024).
-
- Kaiser Permanente Mental health monitoring tool. https://wa-provider.kaiserpermanente.org/static/pdf/provider/patient-ed/... (accessed 20 March 2024).
-
- Nederlands Huisartsen Genootschap (Dutch Society of General Practitioners) NHG guideline on depression. 2019 https://richtlijnen.nhg.org/standaarden/depressie#volledige-tekst-3-beoo... (accessed 20 March 2024).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
