Prevention of bleomycin-induced pulmonary fibrosis by vaccination with the Tocilizumab mimotope
- PMID: 38408907
- PMCID: PMC10900270
- DOI: 10.1080/21645515.2024.2319965
Prevention of bleomycin-induced pulmonary fibrosis by vaccination with the Tocilizumab mimotope
Abstract
Mimotope, a kind of peptide vaccine, is developed to bind natural receptor and inhibit the downstream signaling. We have demonstrated that the vaccination of Tocilizumab mimotopes could alleviate the renal fibrosis by interfering with both IL-6 and ferroptosis signaling. However, the effect of the vaccination of Tocilizumab mimotopes on the fibroblast was not investigated in previous study. Thus, we sought to explore the changes in the fibroblast induced by the Tocilizumab mimotopes vaccination. Bleomycin instillation was performed to construct the pulmonary fibrosis model after the immunization of Tocilizumab mimotopes. Lung histological analysis showed that the Tocilizumab mimotopes could significantly reduce the maladaptive repairment and abnormal remodeling. Immunoblotting assay and fluorescence staining showed that Immunization with the Tocilizumab mimotopes reduces the accumulation of fibrosis-related proteins. High level of lipid peroxidation product was observed in the animal model, while the Tocilizumab mimotopes vaccination could reduce the generation of lipid peroxidation product. Mechanism analysis further showed that Nrf-2 signaling, but not GPX-4 and FSP-1 signaling, was upregulated, and reduced the lipid peroxidation. Our results revealed that in the BLM-induced pulmonary fibrosis, high level of lipid peroxidation product was significantly accumulation in the lung tissues, which might lead to the occurrence of ferroptosis. The IL-6 pathway block therapy could inhibit lipid peroxidation product generation in the lung tissues by upregulating the Nrf-2 signaling, and further alleviate the pulmonary fibrosis.
Keywords: IL-6; Pulmonary fibrosis; Tocilizumab; fibroblast; lipid peroxidation; mimotope.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


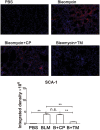



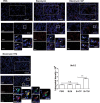

Similar articles
-
Tocilizumab mimotope alleviates kidney injury and fibrosis by inhibiting IL-6 signaling and ferroptosis in UUO model.Life Sci. 2020 Nov 15;261:118487. doi: 10.1016/j.lfs.2020.118487. Epub 2020 Sep 23. Life Sci. 2020. PMID: 32979361
-
Iron-laden macrophage-mediated paracrine profibrotic signaling induces lung fibroblast activation.Am J Physiol Cell Physiol. 2024 Oct 1;327(4):C979-C993. doi: 10.1152/ajpcell.00675.2023. Epub 2024 Aug 26. Am J Physiol Cell Physiol. 2024. PMID: 39183565
-
A recombinant IL-1β vaccine attenuates bleomycin-induced pulmonary fibrosis in mice.Vaccine. 2024 Jul 11;42(18):3774-3788. doi: 10.1016/j.vaccine.2024.04.091. Epub 2024 May 6. Vaccine. 2024. PMID: 38714443
-
Active immunization with Tocilizumab mimotopes induces specific immune responses.BMC Biotechnol. 2015 Jun 2;15:46. doi: 10.1186/s12896-015-0161-9. BMC Biotechnol. 2015. PMID: 26033236 Free PMC article.
-
Zingerone ameliorates oxidative stress and inflammation in bleomycin-induced pulmonary fibrosis: modulation of the expression of TGF-β1 and iNOS.Naunyn Schmiedebergs Arch Pharmacol. 2020 Sep;393(9):1659-1670. doi: 10.1007/s00210-020-01881-7. Epub 2020 May 6. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 32377772
Cited by
-
Ferroptosis: the potential key roles in idiopathic pulmonary fibrosis.Eur J Med Res. 2025 Apr 28;30(1):341. doi: 10.1186/s40001-025-02623-2. Eur J Med Res. 2025. PMID: 40296070 Free PMC article. Review.
References
-
- Sato K, Tsuchiya M, Saldanha J, Koishihara Y, Ohsugi Y, Kishimoto T, Bendig MM.. Reshaping a human antibody to inhibit the interleukin 6-dependent tumor cell growth. Cancer Res. 1993 Feb 15;53(4):851–11. - PubMed
-
- Yokota S, Imagawa T, Mori M, Miyamae T, Aihara Y, Takei S, Iwata N, Umebayashi H, Murata T, Miyoshi M. et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008 Mar 22;371(9617):998–1006. doi:10.1016/S0140-6736(08)60454-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
