Dose of nafamostat mesylate during continuous kidney replacement therapy in critically ill patients: a two-centre observational study
- PMID: 38408970
- PMCID: PMC10895744
- DOI: 10.1186/s12882-024-03506-0
Dose of nafamostat mesylate during continuous kidney replacement therapy in critically ill patients: a two-centre observational study
Abstract
Background: Nafamostat mesylate is an anticoagulant used for critically ill patients during continuous kidney replacement therapy (CKRT), characterised by its short half-life. However, its optimal dosage remains unclear. This study aimed to explore the optimal dosage of nafamostat mesylate during CKRT.
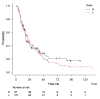
Methods: We conducted a two-centre observational study. We screened all critically ill adult patients who required CKRT in the intensive care unit (ICU) from September 2013 to August 2021; we included patients aged ≥ 18 years who received nafamostat mesylate during CKRT. The primary outcome was filter life, defined as the time from CKRT initiation to the end of the first filter use due to filter clotting. The secondary outcomes included safety and other clinical outcomes. The survival analysis of filter patency by the nafamostat mesylate dosage adjusted for bleeding risk and haemofiltration was performed using a Cox proportional hazards model.
Results: We included 269 patients. The mean dose of nafamostat mesylate was 15.8 mg/hr (Standard deviation (SD), 8.8; range, 5.0 to 30.0), and the median filter life was 18.3 h (Interquartile range (IQR), 9.28 to 36.7). The filter survival analysis showed no significant association between the filter life and nafamostat mesylate dosage (hazard ratio 1.12; 95 CI 0.74-1.69, p = 0.60) after adjustment for bleeding risk and addition of haemofiltration to haemodialysis.
Conclusions: We observed no dose-response relationship between the dose of nafamostat mesylate (range: 5 to 30 mg/h) and the filter life during CKRT in critically ill patients. The optimal dose to prevent filter clotting safely needs further study in randomised controlled trials.
Trial registration: Not applicable.
Keywords: Acute kidney injury; Anticoagulant; Continuous kidney replacement therapy; Dose; Filter life; Nafamostat mesylate.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group KDIGO Clinical Practice Guideline for Acute kidney Injury. Kidney Int Suppl (2011) 2012;2(1):89–115.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical