A phase 1b study of zilovertamab in combination with paclitaxel for locally advanced/unresectable or metastatic Her2-negative breast cancer
- PMID: 38408999
- PMCID: PMC10895766
- DOI: 10.1186/s13058-024-01782-0
A phase 1b study of zilovertamab in combination with paclitaxel for locally advanced/unresectable or metastatic Her2-negative breast cancer
Erratum in
-
Correction: a phase 1b study of zilovertamab in combination with paclitaxel for locally advanced/unresectable or metastatic HER2-negative breast cancer.Breast Cancer Res. 2024 Mar 13;26(1):46. doi: 10.1186/s13058-024-01805-w. Breast Cancer Res. 2024. PMID: 38481291 Free PMC article. No abstract available.
Abstract
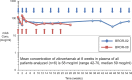
Background: Zilovertamab is a humanized monoclonal antibody targeting ROR1, an onco-embryonic antigen expressed by malignant cells of a variety of solid tumors, including breast cancer. A prior phase 1 study showed that zilovertamab was well tolerated and effective in inhibiting ROR1-signaling, which leads to activation of ERK1/2, NF-κB, and NRF2 target genes. This phase 1b study evaluated the safety and tolerability of zilovertamab with paclitaxel in patients with advanced breast cancer.
Patients and methods: Eligible patients had locally advanced, unresectable, or metastatic HER2- breast cancer with Eastern Cooperative Group performance status of 0-2 and without prior taxane therapy in the advanced setting. Study treatment included 600 mg of zilovertamab administered intravenously (IV) on Days 1 and 15 of Cycle 1 and then Day 1 of each 28-day cycle along with paclitaxel weekly at 80 mg/m2 IV.
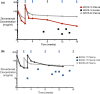
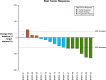
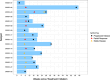
Results: Study patients had received a median of 4 prior therapies (endocrine therapy + chemotherapy) for locally advanced, unresectable, or metastatic disease. No patient discontinued therapy due to toxicity ascribed to zilovertamab. Adverse events were consistent with the known safety profile of paclitaxel. Of 16 patients, 6 (38%) had a partial response, and 6/16 (38%) patients had stable disease as best tumor response.
Conclusion: The combination of zilovertamab and paclitaxel was safe and well tolerated in heavily pre-treated advanced breast cancer patients. Further evaluation of ROR1 targeting in breast cancer patients with zilovertamab is warranted.
Trial registration: NCT02776917. Registered on ClinicalTrials.gov on 05/17/2016.
Keywords: Metastatic breast cancer; Paclitaxel; ROR1; Zilovertamab.
© 2024. The Author(s).
Conflict of interest statement
R.A.S: Astra Zeneca (advisory board), Gilead (speaker’s bureau), Quantum Leap (consultant), Stemline (advisory board). Institutional Funding from OBI Pharmaceuticals, Quantum Leap, Astra Zeneca, and Gilead. ORCID profile: 0000-0003-2289-6234. H.B.S.: MJH Life Sciences (consultant). ORCID profile: 0009-0003-8700-1451. T.H.: No conflicts of interest. R.B.S.: Genentech, Inc. (current employee). Dr. Schwab was affiliated with the University of California San Diego Department of Medicine and Moores Cancer Center at the time this trial was conducted. ORCID profile: 0000-0002-0488-1226. E.I.P.: No conflicts of interest. M.P.: No conflicts of interest. E.W.: No conflicts of interest. E.M.G.: No conflicts of interest. ORCID profile: 0000-0002-6060-6106. L.Z.R.: No conflicts of interest. A.M.: No conflicts of interest. B.C.: No conflicts of interest. J.B.B.: Oncternal Therapeutics, Inc. (employee, Board director, stock owner, stock option holder). G.F.W. II: No conflicts of interest. K.M.: No conflicts of interest. C.J.: Dr. Jamieson is a co-founder of Aspera Biomedicines and receives royalties from Stanford University for patents related to Forty Seven, Inc. T.J.K.: Zilovertamab was developed by T.J.K. and his laboratory and licensed by the University of California to Oncternal Therapeutics, Inc. and VelosBio, Inc. which provided stock and research funding. ORCID profile: 0000-0002-0064-4549. B.A.P.: Dr. Barbara Parker has stock from Merck and Bioatla (spouse), consulting role with Bioatla (spouse), Samumed LLC (spouse), and Dare Biosciences, research funding to her institution from Glaxo/SmithKline, Genentech/Roche, Novartis, Pfizer, and Oncternal Therapeutics and receives royalties from Salk Institute (spouse). ORCID profile: 000-0003-0499-1289.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

