Inhibition of growth of hepatocellular carcinoma by co-delivery of anti-PD-1 antibody and sorafenib using biomimetic nano-platelets
- PMID: 38409035
- PMCID: PMC10898182
- DOI: 10.1186/s12885-024-12006-1
Inhibition of growth of hepatocellular carcinoma by co-delivery of anti-PD-1 antibody and sorafenib using biomimetic nano-platelets
Abstract
Background: Traditional nanodrug delivery systems have some limitations, such as eliciting immune responses and inaccuracy in targeting tumor microenvironments.
Materials and methods: Targeted drugs (Sorafenib, Sora) nanometers (hollow mesoporous silicon, HMSN) were designed, and then coated with platelet membranes to form aPD-1-PLTM-HMSNs@Sora to enhance the precision of drug delivery systems to the tumor microenvironment, so that more effective immunotherapy was achieved.
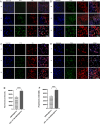
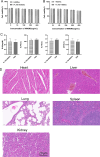
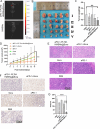
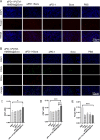
Results: These biomimetic nanoparticles were validated to have the same abilities as platelet membranes (PLTM), including evading the immune system. The successful coating of HMSNs@Sora with PLTM was corroborated by transmission electron microscopy (TEM), western blot and confocal laser microscopy. The affinity of aPD-1-PLTM-HMSNs@Sora to tumor cells was stronger than that of HMSNs@Sora. After drug-loaded particles were intravenously injected into hepatocellular carcinoma model mice, they were demonstrated to not only directly activate toxic T cells, but also increase the triggering release of Sora. The combination of targeted therapy and immunotherapy was found to be of gratifying antineoplastic function on inhibiting primary tumor growth.
Conclusions: The aPD-1-PLTM-HMSNs@Sora nanocarriers that co-delivery of aPD-1 and Sorafenib integrates unique biomimetic properties and excellent targeting performance, and provides a neoteric idea for drug delivery of personalized therapy for primary hepatocellular carcinoma (HCC).
Keywords: Immunotherapy; Nanoparticles; Platelet membrane; Sorafenib; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Transferrin receptor-targeted HMSN for sorafenib delivery in refractory differentiated thyroid cancer therapy.Int J Nanomedicine. 2018 Dec 6;13:8339-8354. doi: 10.2147/IJN.S187240. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30584304 Free PMC article.
-
Targeted co-delivery of PD-L1 monoclonal antibody and sorafenib to circulating tumor cells via platelet-functionalized nanocarriers.Biochem Biophys Res Commun. 2023 Sep 3;671:335-342. doi: 10.1016/j.bbrc.2023.05.124. Epub 2023 Jun 10. Biochem Biophys Res Commun. 2023. PMID: 37327705
-
Preparation and characterization of parthenolide nanocrystals for enhancing therapeutic effects of sorafenib against advanced hepatocellular carcinoma.Int J Pharm. 2020 Jun 15;583:119375. doi: 10.1016/j.ijpharm.2020.119375. Epub 2020 Apr 25. Int J Pharm. 2020. PMID: 32344021
-
Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma.Theranostics. 2021 Mar 13;11(11):5464-5490. doi: 10.7150/thno.54822. eCollection 2021. Theranostics. 2021. PMID: 33859758 Free PMC article. Review.
-
Research progress of sorafenib drug delivery system in the treatment of hepatocellular carcinoma: An update.Biomed Pharmacother. 2024 Aug;177:117118. doi: 10.1016/j.biopha.2024.117118. Epub 2024 Jul 14. Biomed Pharmacother. 2024. PMID: 39002440 Review.
Cited by
-
Nanomaterials in the diagnosis and treatment of gastrointestinal tumors: New clinical choices and treatment strategies.Mater Today Bio. 2025 Apr 19;32:101782. doi: 10.1016/j.mtbio.2025.101782. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40331152 Free PMC article. Review.
-
Application of nanotechnology in the treatment of hepatocellular carcinoma.Front Pharmacol. 2024 Nov 29;15:1438819. doi: 10.3389/fphar.2024.1438819. eCollection 2024. Front Pharmacol. 2024. PMID: 39679376 Free PMC article. Review.
-
Precision nanomedicine: navigating the tumor microenvironment for enhanced cancer immunotherapy and targeted drug delivery.Mol Cancer. 2025 Jun 3;24(1):160. doi: 10.1186/s12943-025-02357-z. Mol Cancer. 2025. PMID: 40457437 Free PMC article. Review.
-
Development of a risk prediction nomogram model of pyrotinib-induced severe diarrhea.BMC Cancer. 2025 Jan 10;25(1):59. doi: 10.1186/s12885-025-13427-2. BMC Cancer. 2025. PMID: 39794736 Free PMC article.
-
Nanomedicines in the Treatment of Liver Fibrosis: A Review.Int J Nanomedicine. 2025 Aug 5;20:9641-9665. doi: 10.2147/IJN.S524078. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40785947 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

