Updates on the role of epigenetics in familial mediterranean fever (FMF)
- PMID: 38409042
- PMCID: PMC10898143
- DOI: 10.1186/s13023-024-03098-w
Updates on the role of epigenetics in familial mediterranean fever (FMF)
Abstract
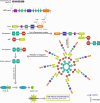
Familial Mediterranean Fever (FMF) is an autosomal recessive autoinflammatory disease caused by mutations in the MEFV (MEditerranean FeVer) gene that affects people originating from the Mediterranean Sea. The high variability in severity and clinical manifestations observed not only between ethnic groups but also between and within families is mainly related to MEFV allelic heterogeneity and to some modifying genes. In addition to the genetic factors underlying FMF, the environment plays a significant role in the development and manifestation of this disease through various epigenetic mechanisms, including DNA methylation, histone modification, and noncoding RNAs. Indeed, epigenetic events have been identified as an important pathophysiological determinant of FMF and co-factors shaping the clinical picture and outcome of the disease. Therefore, it is essential to better understand the contribution of epigenetic factors to autoinflammatory diseases, namely, FMF, to improve disease prognosis and potentially develop effective targeted therapies. In this review, we highlight the latest updates on the role of epigenetics in FMF.
Keywords: DNA methylation; Epigenetics; Familial Mediterranean Fever (FMF); Histone modification; miRNA.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
Similar articles
-
Epigenetic insights into Familial Mediterranean Fever: Increased RGS10 expression and histone modifications accompanies inflammation in familial Mediterranean fever disease.Gene. 2024 May 15;906:148222. doi: 10.1016/j.gene.2024.148222. Epub 2024 Feb 6. Gene. 2024. PMID: 38331118
-
NLRP13 inflammasome complex is hypermethylated in familial Mediterranean fever and global methylation correlates with the disease severity.Ann Hum Genet. 2023 May;87(3):115-124. doi: 10.1111/ahg.12493. Epub 2022 Dec 30. Ann Hum Genet. 2023. PMID: 36583259
-
miRNAs as attractive diagnostic and therapeutic targets for Familial Mediterranean Fever.Mod Rheumatol. 2021 Sep;31(5):949-959. doi: 10.1080/14397595.2020.1868674. Epub 2021 Feb 2. Mod Rheumatol. 2021. PMID: 33427536
-
Familial Mediterranean fever and microRNAs.Int J Immunogenet. 2023 Dec;50(6):273-280. doi: 10.1111/iji.12640. Epub 2023 Oct 4. Int J Immunogenet. 2023. PMID: 37794570 Review.
-
[Familial Mediterranean Fever (FMF): from diagnosis to treatment].Sante. 2004 Oct-Dec;14(4):261-6. Sante. 2004. PMID: 15745878 Review. French.
Cited by
-
Updates on the molecular spectrum of MEFV variants in lebanese patients with Familial Mediterranean Fever.Front Genet. 2025 Jan 17;15:1506656. doi: 10.3389/fgene.2024.1506656. eCollection 2024. Front Genet. 2025. PMID: 39897620 Free PMC article.
-
Molecular analyses of MEFV gene mutation variants in Turkish population.Mol Biol Rep. 2024 Jul 23;51(1):844. doi: 10.1007/s11033-024-09786-x. Mol Biol Rep. 2024. PMID: 39042260
-
Enigmatic link between familial mediterranean fever and dietary components: a novel approach to personalized nutrition.BMC Nutr. 2025 Apr 25;11(1):85. doi: 10.1186/s40795-025-01071-9. BMC Nutr. 2025. PMID: 40281641 Free PMC article.
-
A narrative review on the role of cytokines in the pathogenesis and treatment of familial Mediterranean fever: an emphasis on pediatric cases.Front Pediatr. 2024 Jul 26;12:1421353. doi: 10.3389/fped.2024.1421353. eCollection 2024. Front Pediatr. 2024. PMID: 39132307 Free PMC article. Review.
-
Familial Mediterranean Fever in Childhood.Turk Arch Pediatr. 2024 Nov 1;59(6):527-534. doi: 10.5152/TurkArchPediatr.2024.24188. Turk Arch Pediatr. 2024. PMID: 39540697 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

